New tools could catch disease outbreaks earlier - or predict them

A team at the Catalan Institution for Research and Advanced Studies is utilizing drones and weather stations to collect data on how mosquito breeding patterns are changing in response to climate shifts.
Every year, the villages which lie in the so-called ‘Nipah belt’— which stretches along the western border between Bangladesh and India, brace themselves for the latest outbreak. For since 1998, when Nipah virus—a form of hemorrhagic fever most common in Bangladesh—first spilled over into humans, it has been a grim annual visitor to the people of this region.
With a 70 percent fatality rate, no vaccine, and no known treatments, Nipah virus has been dubbed in the Western world as ‘the worst disease no one has ever heard of.’ Currently, outbreaks tend to be relatively contained because it is not very transmissible. The virus circulates throughout Asia in fruit eating bats, and only tends to be passed on to people who consume contaminated date palm sap, a sweet drink which is harvested across Bangladesh.
But as SARS-CoV-2 has shown the world, this can quickly change.
“Nipah virus is among what virologists call ‘the Big 10,’ along with things like Lassa fever and Crimean Congo hemorrhagic fever,” says Noam Ross, a disease ecologist at New York-based non-profit EcoHealth Alliance. “These are pretty dangerous viruses from a lethality perspective, which don’t currently have the capacity to spread into broader human populations. But that can evolve, and you could very well see a variant emerge that has human-human transmission capability.”
That’s not an overstatement. Surveys suggest that mammals harbour about 40,000 viruses, with roughly a quarter capable of infecting humans. The vast majority never get a chance to do so because we don’t encounter them, but climate change can alter that. Recent studies have found that as animals relocate to new habitats due to shifting environmental conditions, the coming decades will bring around 300,000 first encounters between species which normally don’t interact, especially in tropical Africa and southeast Asia. All these interactions will make it far more likely for hitherto unknown viruses to cross paths with humans.
That’s why for the last 16 years, EcoHealth Alliance has been conducting ongoing viral surveillance projects across Bangladesh. The goal is to understand why Nipah is so much more prevalent in the western part of the country, compared to the east, and keep a watchful eye out for new Nipah strains as well as other dangerous pathogens like Ebola.
"There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them," says Cat Lippi, medical geography researcher at the University of Florida.
Until very recently this kind of work has been hampered by the limitations of viral surveillance technology. The PREDICT project, a $200 million initiative funded by the United States Agency for International Development, which conducted surveillance across the Amazon Basin, Congo Basin and extensive parts of South and Southeast Asia, relied upon so-called nucleic acid assays which enabled scientists to search for the genetic material of viruses in animal samples.
However, the project came under criticism for being highly inefficient. “That approach requires a big sampling effort, because of the rarity of individual infections,” says Ross. “Any particular animal may be infected for a couple of weeks, maybe once or twice in its lifetime. So if you sample thousands and thousands of animals, you'll eventually get one that has an Ebola virus infection right now.”
Ross explains that there is now far more interest in serological sampling—the scientific term for the process of drawing blood for antibody testing. By searching for the presence of antibodies in the blood of humans and animals, scientists have a greater chance of detecting viruses which started circulating recently.
Despite the controversy surrounding EcoHealth Alliance’s involvement in so-called gain of function research—experiments that study whether viruses might mutate into deadlier strains—the organization’s separate efforts to stay one step ahead of pathogen evolution are key to stopping the next pandemic.
“Having really cheap and fast surveillance is really important,” says Ross. “Particularly in a place where there's persistent, low level, moderate infections that potentially have the ability to develop into more epidemic or pandemic situations. It means there’s a pathway that something more dangerous can come through."
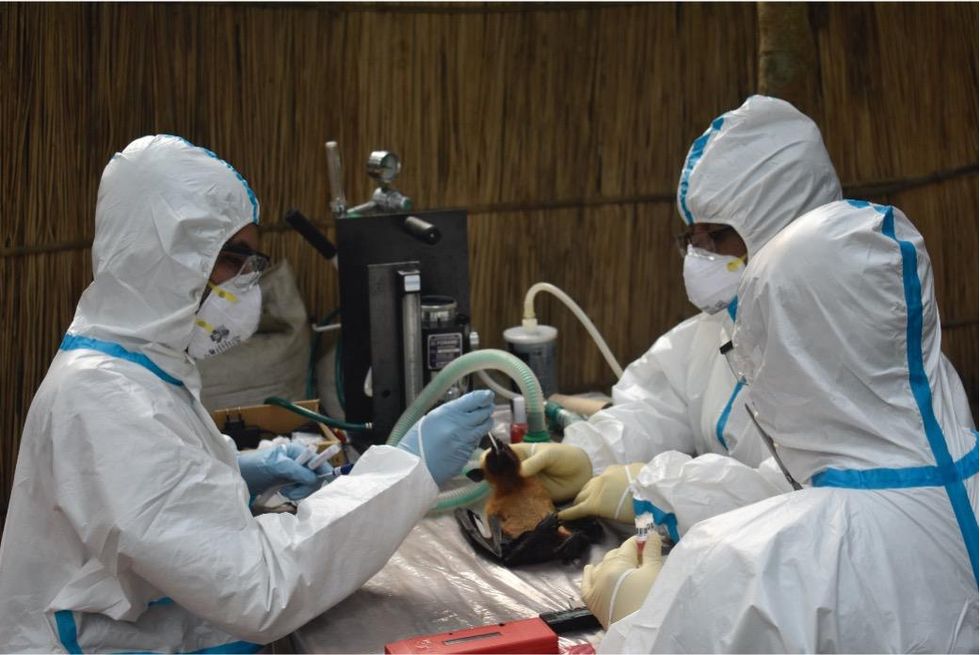
Scientists are searching for the presence of antibodies in the blood of humans and animals in hopes to detect viruses that recently started circulating.
EcoHealth Alliance
In Bangladesh, EcoHealth Alliance is attempting to do this using a newer serological technology known as a multiplex Luminex assay, which tests samples against a panel of known antibodies against many different viruses. It collects what Ross describes as a ‘footprint of information,’ which allows scientists to tell whether the sample contains the presence of a known pathogen or something completely different and needs to be investigated further.
By using this technology to sample human and animal populations across the country, they hope to gain an idea of whether there are any novel Nipah virus variants or strains from the same family, as well as other deadly viral families like Ebola.
This is just one of several novel tools being used for viral discovery in surveillance projects around the globe. Multiple research groups are taking PREDICT’s approach of looking for novel viruses in animals in various hotspots. They collect environmental DNA—mucus, faeces or shed skin left behind in soil, sediment or water—which can then be genetically sequenced.
Five years ago, this would have been a painstaking work requiring bringing collected samples back to labs. Today, thanks to the vast amounts of money spent on new technologies during COVID-19, researchers now have portable sequencing tools they can take out into the field.
Christopher Jerde, a researcher at the UC Santa Barbara Marine Science Institute, points to the Oxford Nanopore MinION sequencer as one example. “I tried one of the early versions of it four years ago, and it was miserable,” he says. “But they’ve really improved, and what we’re going to be able to do in the next five to ten years will be amazing. Instead of having to carefully transport samples back to the lab, we're going to have cigar box-shaped sequencers that we take into the field, plug into a laptop, and do the whole sequencing of an organism.”
In the past, viral surveillance has had to be very targeted and focused on known families of viruses, potentially missing new, previously unknown zoonotic pathogens. Jerde says that the rise of portable sequencers will lead to what he describes as “true surveillance.”
“Before, this was just too complex,” he says. “It had to be very focused, for example, looking for SARS-type viruses. Now we’re able to say, ‘Tell us all the viruses that are here?’ And this will give us true surveillance – we’ll be able to see the diversity of all the pathogens which are in these spots and have an understanding of which ones are coming into the population and causing damage.”
But being able to discover more viruses also comes with certain challenges. Some scientists fear that the speed of viral discovery will soon outpace the human capacity to analyze them all and assess the threat that they pose to us.
“I think we're already there,” says Jason Ladner, assistant professor at Northern Arizona University’s Pathogen and Microbiome Institute. “If you look at all the papers on the expanding RNA virus sphere, there are all of these deposited partial or complete viral sequences in groups that we just don't know anything really about yet.” Bats, for example, carry a myriad of viruses, whose ability to infect human cells we understand very poorly.
Cultivating these viruses under laboratory conditions and testing them on organoids— miniature, simplified versions of organs created from stem cells—can help with these assessments, but it is a slow and painstaking work. One hope is that in the future, machine learning could help automate this process. The new SpillOver Viral Risk Ranking platform aims to assess the risk level of a given virus based on 31 different metrics, while other computer models have tried to do the same based on the similarity of a virus’s genomic sequence to known zoonotic threats.
However, Ladner says that these types of comparisons are still overly simplistic. For one thing, scientists are still only aware of a few hundred zoonotic viruses, which is a very limited data sample for accurately assessing a novel pathogen. Instead, he says that there is a need for virologists to develop models which can determine viral compatibility with human cells, based on genomic data.
“One thing which is really useful, but can be challenging to do, is understand the cell surface receptors that a given virus might use,” he says. “Understanding whether a virus is likely to be able to use proteins on the surface of human cells to gain entry can be very informative.”
As the Earth’s climate heats up, scientists also need to better model the so-called vector borne diseases such as dengue, Zika, chikungunya and yellow fever. Transmitted by the Aedes mosquito residing in humid climates, these blights currently disproportionally affect people in low-income nations. But predictions suggest that as the planet warms and the pests find new homes, an estimated one billion people who currently don’t encounter them might be threatened by their bites by 2080. “When it comes to mosquito-borne diseases we have to worry about shifts in suitable habitat,” says Cat Lippi, a medical geography researcher at the University of Florida. “As climate patterns change on these big scales, we expect to see shifts in where people will be at risk for contracting these diseases.”
Public health practitioners and government decision-makers need tools to make climate-informed decisions about the evolving threat of different infectious diseases. Some projects are already underway. An ongoing collaboration between the Catalan Institution for Research and Advanced Studies and researchers in Brazil and Peru is utilizing drones and weather stations to collect data on how mosquitoes change their breeding patterns in response to climate shifts. This information will then be fed into computer algorithms to predict the impact of mosquito-borne illnesses on different regions.

The team at the Catalan Institution for Research and Advanced Studies is using drones and weather stations to collect data on how mosquito breeding patterns change due to climate shifts.
Gabriel Carrasco
Lippi says that similar models are urgently needed to predict how changing climate patterns affect respiratory, foodborne, waterborne and soilborne illnesses. The UK-based Wellcome Trust has allocated significant assets to fund such projects, which should allow scientists to monitor the impact of climate on a much broader range of infections. “There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them,” she says.
COVID-19’s havoc boosted funding for infectious disease research, but as its threats begin to fade from policymakers’ focus, the money may dry up. Meanwhile, scientists warn that another major infectious disease outbreak is inevitable, potentially within the next decade, so combing the planet for pathogens is vital. “Surveillance is ultimately a really boring thing that a lot of people don't want to put money into, until we have a wide scale pandemic,” Jerde says, but that vigilance is key to thwarting the next deadly horror. “It takes a lot of patience and perseverance to keep looking.”
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are increasing vulnerabilities to infectious diseases by land and by sea. The magazine probes how scientists are making progress with leaders in other fields toward solutions that embrace diverse perspectives and the interconnectedness of all lifeforms and the planet.
The Pandemic Is Ushering in a More Modern—and Ethical—Way of Studying New Drugs and Diseases
Miniature lab-grown models of human organs have major advantages over standard animal testing.
Before the onset of the coronavirus pandemic, Dutch doctoral researcher Joep Beumer had used miniature lab-grown organs to study the human intestine as part of his PhD thesis. When lockdown hit, however, he was forced to delay his plans for graduation. Overwhelmed by a sense of boredom after the closure of his lab at the Hubrecht Institute, in the Netherlands, he began reading literature related to COVID-19.
"By February [2020], there were already reports on coronavirus symptoms in the intestinal tract," Beumer says, adding that this piqued his interest. He wondered if he could use his miniature models – called organoids -- to study how the coronavirus infects the intestines.
But he wasn't the only one to follow this train of thought. In the year since the pandemic began, many researchers have been using organoids to study how the coronavirus infects human cells, and find potential treatments. Beumer's pivot represents a remarkable and fast-emerging paradigm shift in how drugs and diseases will be studied in the coming decades. With future pandemics likely to be more frequent and deadlier, such a shift is necessary to reduce the average clinical development time of 5.9 years for antiviral agents.
Part of that shift means developing models that replicate human biology in the lab. Animal models, which are the current standard in biomedical research, fail to do so—96% of drugs that pass animal testing, for example, fail to make it to market. Injecting potentially toxic drugs into living creatures, before eventually slaughtering them, also raises ethical concerns for some. Organoids, on the other hand, respond to infectious diseases, or potential treatments, in a way that is relevant to humans, in addition to being slaughter-free.

Human intestinal organoids infected with SARS-CoV-2 (white).
Credit: Joep Beumer/Clevers group/Hubrecht Institute
Urgency Sparked Momentum
Though brain organoids were previously used to study the Zika virus during the 2015-16 epidemic, it wasn't until COVID-19 that the field really started to change. "The organoid field has advanced a lot in the last year. The speed at which it happened is crazy," says Shuibing Chen, an associate professor at Weill Cornell Medicine in New York. She adds that many federal and private funding agencies have now seen the benefits of organoids, and are starting to appreciate their potential in the biomedical field.
Last summer, the Organo-Strat (OS) network—a German network that uses human organoid models to study COVID-19's effects—received 3.2 million euros in funding from the German government. "When the pandemic started, we became aware that we didn't have the right models to immediately investigate the effects of the virus," says Andreas Hocke, professor of infectious diseases at the Charité Universitätsmedizin in Berlin, Germany, and coordinator of the OS network. Hocke explained that while the World Health Organization's animal models showed an "overlap of symptoms'' with humans, there was "no clear reflection" of the same disease.
"The network functions as a way of connecting organoid experts with infectious disease experts across Germany," Hocke continues. "Having organoid models on demand means we can understand how a virus infects human cells from the first moment it's isolated." Overall, OS aims to create infrastructure that could be applied to future pandemics. There are 28 sub-projects involved in the network, covering a wide assortment of individual organoids.
Cost, however, remains an obstacle to scaling up, says Chen. She says there is also a limit to what we can learn from organoids, given that they only represent a single organ. "We can add drugs to organoids to see how the cells respond, but these tests don't tell us anything about drug metabolism, for example," she explains.
A Related "Leaps" in Progress
One way to solve this issue is to use an organ-on-a-chip system. These are miniature chips containing a variety of human cells, as well as small channels along which functions like blood or air flow can be recreated. This allows scientists to perform more complex experiments, like studying drug metabolism, while producing results that are relevant to humans.

An organ-on-a-chip system.
Credit: Fraunhofer IGB
Such systems are also able to elicit an immune response. The FDA has even entered into an agreement with Wyss Institute spinoff Emulate to use their lung-on-a-chip system to test COVID-19 vaccines. Representing multiple organs in one system is also possible. Berlin-based TissUse are aiming to make a so-called 'human on a chip' system commercially available. But TissUse senior scientist Ilka Maschmeyer warns that there is a limit to how far the technology can go. "The system will not think or feel, so it wouldn't be possible to test for illnesses affecting these abilities," she says.
Some challenges also remain in the usability of organs-on-a-chip. "Specialized training is required to use them as they are so complex," says Peter Loskill, assistant professor and head of the organ-on-a-chip group at the University of Tübingen, Germany. Hocke agrees with this. "Cell culture scientists would easily understand how to use organoids in a lab, but when using a chip, you need additional biotechnology knowledge," he says.
One major advantage of both technologies is the possibility of personalized medicine: Cells can be taken from a patient and put onto a chip, for example, to test their individual response to a treatment. Loskill also says there are other uses outside of the biomedical field, such as cosmetic and chemical testing.
"Although these technologies offer a lot of possibilities, they need time to develop," Loskill continues. He stresses, however, that it's not just the technology that needs to change. "There's a lot of conservative thinking in biomedical research that says this is how we've always done things. To really study human biology means approaching research questions in a completely new way."
Even so, he thinks that the pandemic marked a shift in people's thinking—no one cared how the results were found, as long as it was done quickly. But Loskill adds that it's important to balance promise, potential, and expectations when it comes to these new models. "Maybe in 15 years' time we will have a limited number of animal models in comparison to now, but the timescale depends on many factors," he says.
Beumer, now a post-doc, was eventually allowed to return to the lab to develop his coronavirus model, and found working on it to be an eye-opening experience. He saw first-hand how his research could have an impact on something that was affecting the entire human race, as well as the pressure that comes with studying potential treatments. Though he doesn't see a future for himself in infectious diseases, he hopes to stick with organoids. "I've now gotten really excited about the prospect of using organoids for drug discovery," he says.
The coronavirus pandemic has slowed society down in many respects, but it has flung biomedical research into the future—from mRNA vaccines to healthcare models based on human biology. It may be difficult to fully eradicate animal models, but over the coming years, organoids and organs-on-a-chip may become the standard for the sake of efficacy -- and ethics.
Jack McGovan is a freelance science writer based in Berlin. His main interests center around sustainability, food, and the multitude of ways in which the human world intersects with animal life. Find him on Twitter @jack_mcgovan."
New Podcast: Why Dr. Ashish Jha Expects a Good Summer
Dr. Jha discusses Covid vaccine passports, how supply and demand of the vaccines is about to shift, the AstraZeneca situation, what's new with kids, herd immunity, and more.
Making Sense of Science features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
Hear the 30-second trailer:
Listen to the whole episode: "Why Dr. Ashish Jha Expects a Good Summer"
Dr. Ashish Jha, dean of public health at Brown University, discusses the latest developments around the Covid-19 vaccines, including supply and demand, herd immunity, kids, vaccine passports, and why he expects the summer to look very good.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.