New tools could catch disease outbreaks earlier - or predict them

A team at the Catalan Institution for Research and Advanced Studies is utilizing drones and weather stations to collect data on how mosquito breeding patterns are changing in response to climate shifts.
Every year, the villages which lie in the so-called ‘Nipah belt’— which stretches along the western border between Bangladesh and India, brace themselves for the latest outbreak. For since 1998, when Nipah virus—a form of hemorrhagic fever most common in Bangladesh—first spilled over into humans, it has been a grim annual visitor to the people of this region.
With a 70 percent fatality rate, no vaccine, and no known treatments, Nipah virus has been dubbed in the Western world as ‘the worst disease no one has ever heard of.’ Currently, outbreaks tend to be relatively contained because it is not very transmissible. The virus circulates throughout Asia in fruit eating bats, and only tends to be passed on to people who consume contaminated date palm sap, a sweet drink which is harvested across Bangladesh.
But as SARS-CoV-2 has shown the world, this can quickly change.
“Nipah virus is among what virologists call ‘the Big 10,’ along with things like Lassa fever and Crimean Congo hemorrhagic fever,” says Noam Ross, a disease ecologist at New York-based non-profit EcoHealth Alliance. “These are pretty dangerous viruses from a lethality perspective, which don’t currently have the capacity to spread into broader human populations. But that can evolve, and you could very well see a variant emerge that has human-human transmission capability.”
That’s not an overstatement. Surveys suggest that mammals harbour about 40,000 viruses, with roughly a quarter capable of infecting humans. The vast majority never get a chance to do so because we don’t encounter them, but climate change can alter that. Recent studies have found that as animals relocate to new habitats due to shifting environmental conditions, the coming decades will bring around 300,000 first encounters between species which normally don’t interact, especially in tropical Africa and southeast Asia. All these interactions will make it far more likely for hitherto unknown viruses to cross paths with humans.
That’s why for the last 16 years, EcoHealth Alliance has been conducting ongoing viral surveillance projects across Bangladesh. The goal is to understand why Nipah is so much more prevalent in the western part of the country, compared to the east, and keep a watchful eye out for new Nipah strains as well as other dangerous pathogens like Ebola.
"There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them," says Cat Lippi, medical geography researcher at the University of Florida.
Until very recently this kind of work has been hampered by the limitations of viral surveillance technology. The PREDICT project, a $200 million initiative funded by the United States Agency for International Development, which conducted surveillance across the Amazon Basin, Congo Basin and extensive parts of South and Southeast Asia, relied upon so-called nucleic acid assays which enabled scientists to search for the genetic material of viruses in animal samples.
However, the project came under criticism for being highly inefficient. “That approach requires a big sampling effort, because of the rarity of individual infections,” says Ross. “Any particular animal may be infected for a couple of weeks, maybe once or twice in its lifetime. So if you sample thousands and thousands of animals, you'll eventually get one that has an Ebola virus infection right now.”
Ross explains that there is now far more interest in serological sampling—the scientific term for the process of drawing blood for antibody testing. By searching for the presence of antibodies in the blood of humans and animals, scientists have a greater chance of detecting viruses which started circulating recently.
Despite the controversy surrounding EcoHealth Alliance’s involvement in so-called gain of function research—experiments that study whether viruses might mutate into deadlier strains—the organization’s separate efforts to stay one step ahead of pathogen evolution are key to stopping the next pandemic.
“Having really cheap and fast surveillance is really important,” says Ross. “Particularly in a place where there's persistent, low level, moderate infections that potentially have the ability to develop into more epidemic or pandemic situations. It means there’s a pathway that something more dangerous can come through."
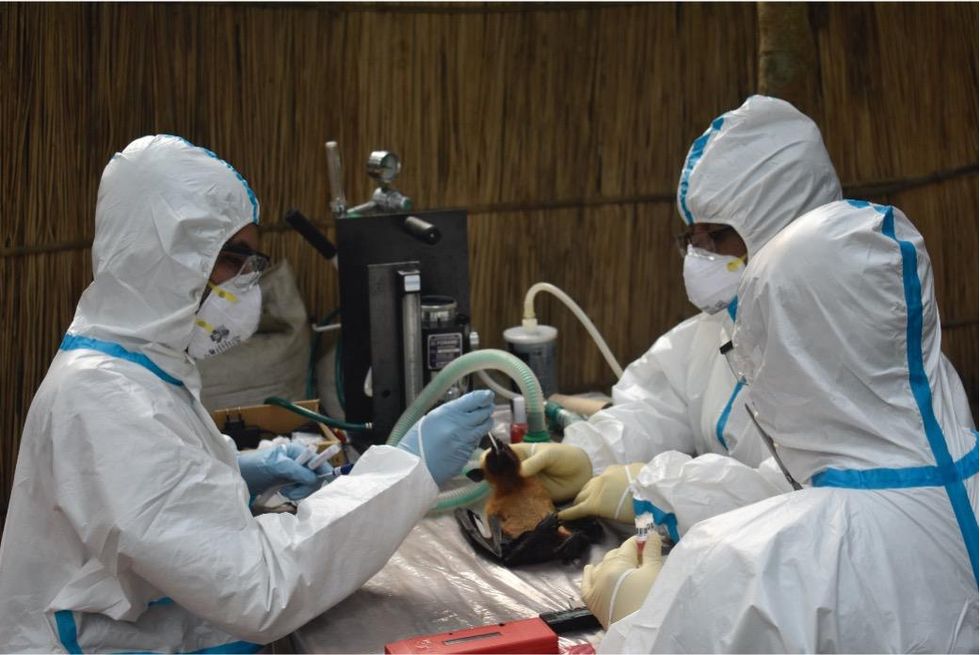
Scientists are searching for the presence of antibodies in the blood of humans and animals in hopes to detect viruses that recently started circulating.
EcoHealth Alliance
In Bangladesh, EcoHealth Alliance is attempting to do this using a newer serological technology known as a multiplex Luminex assay, which tests samples against a panel of known antibodies against many different viruses. It collects what Ross describes as a ‘footprint of information,’ which allows scientists to tell whether the sample contains the presence of a known pathogen or something completely different and needs to be investigated further.
By using this technology to sample human and animal populations across the country, they hope to gain an idea of whether there are any novel Nipah virus variants or strains from the same family, as well as other deadly viral families like Ebola.
This is just one of several novel tools being used for viral discovery in surveillance projects around the globe. Multiple research groups are taking PREDICT’s approach of looking for novel viruses in animals in various hotspots. They collect environmental DNA—mucus, faeces or shed skin left behind in soil, sediment or water—which can then be genetically sequenced.
Five years ago, this would have been a painstaking work requiring bringing collected samples back to labs. Today, thanks to the vast amounts of money spent on new technologies during COVID-19, researchers now have portable sequencing tools they can take out into the field.
Christopher Jerde, a researcher at the UC Santa Barbara Marine Science Institute, points to the Oxford Nanopore MinION sequencer as one example. “I tried one of the early versions of it four years ago, and it was miserable,” he says. “But they’ve really improved, and what we’re going to be able to do in the next five to ten years will be amazing. Instead of having to carefully transport samples back to the lab, we're going to have cigar box-shaped sequencers that we take into the field, plug into a laptop, and do the whole sequencing of an organism.”
In the past, viral surveillance has had to be very targeted and focused on known families of viruses, potentially missing new, previously unknown zoonotic pathogens. Jerde says that the rise of portable sequencers will lead to what he describes as “true surveillance.”
“Before, this was just too complex,” he says. “It had to be very focused, for example, looking for SARS-type viruses. Now we’re able to say, ‘Tell us all the viruses that are here?’ And this will give us true surveillance – we’ll be able to see the diversity of all the pathogens which are in these spots and have an understanding of which ones are coming into the population and causing damage.”
But being able to discover more viruses also comes with certain challenges. Some scientists fear that the speed of viral discovery will soon outpace the human capacity to analyze them all and assess the threat that they pose to us.
“I think we're already there,” says Jason Ladner, assistant professor at Northern Arizona University’s Pathogen and Microbiome Institute. “If you look at all the papers on the expanding RNA virus sphere, there are all of these deposited partial or complete viral sequences in groups that we just don't know anything really about yet.” Bats, for example, carry a myriad of viruses, whose ability to infect human cells we understand very poorly.
Cultivating these viruses under laboratory conditions and testing them on organoids— miniature, simplified versions of organs created from stem cells—can help with these assessments, but it is a slow and painstaking work. One hope is that in the future, machine learning could help automate this process. The new SpillOver Viral Risk Ranking platform aims to assess the risk level of a given virus based on 31 different metrics, while other computer models have tried to do the same based on the similarity of a virus’s genomic sequence to known zoonotic threats.
However, Ladner says that these types of comparisons are still overly simplistic. For one thing, scientists are still only aware of a few hundred zoonotic viruses, which is a very limited data sample for accurately assessing a novel pathogen. Instead, he says that there is a need for virologists to develop models which can determine viral compatibility with human cells, based on genomic data.
“One thing which is really useful, but can be challenging to do, is understand the cell surface receptors that a given virus might use,” he says. “Understanding whether a virus is likely to be able to use proteins on the surface of human cells to gain entry can be very informative.”
As the Earth’s climate heats up, scientists also need to better model the so-called vector borne diseases such as dengue, Zika, chikungunya and yellow fever. Transmitted by the Aedes mosquito residing in humid climates, these blights currently disproportionally affect people in low-income nations. But predictions suggest that as the planet warms and the pests find new homes, an estimated one billion people who currently don’t encounter them might be threatened by their bites by 2080. “When it comes to mosquito-borne diseases we have to worry about shifts in suitable habitat,” says Cat Lippi, a medical geography researcher at the University of Florida. “As climate patterns change on these big scales, we expect to see shifts in where people will be at risk for contracting these diseases.”
Public health practitioners and government decision-makers need tools to make climate-informed decisions about the evolving threat of different infectious diseases. Some projects are already underway. An ongoing collaboration between the Catalan Institution for Research and Advanced Studies and researchers in Brazil and Peru is utilizing drones and weather stations to collect data on how mosquitoes change their breeding patterns in response to climate shifts. This information will then be fed into computer algorithms to predict the impact of mosquito-borne illnesses on different regions.

The team at the Catalan Institution for Research and Advanced Studies is using drones and weather stations to collect data on how mosquito breeding patterns change due to climate shifts.
Gabriel Carrasco
Lippi says that similar models are urgently needed to predict how changing climate patterns affect respiratory, foodborne, waterborne and soilborne illnesses. The UK-based Wellcome Trust has allocated significant assets to fund such projects, which should allow scientists to monitor the impact of climate on a much broader range of infections. “There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them,” she says.
COVID-19’s havoc boosted funding for infectious disease research, but as its threats begin to fade from policymakers’ focus, the money may dry up. Meanwhile, scientists warn that another major infectious disease outbreak is inevitable, potentially within the next decade, so combing the planet for pathogens is vital. “Surveillance is ultimately a really boring thing that a lot of people don't want to put money into, until we have a wide scale pandemic,” Jerde says, but that vigilance is key to thwarting the next deadly horror. “It takes a lot of patience and perseverance to keep looking.”
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are increasing vulnerabilities to infectious diseases by land and by sea. The magazine probes how scientists are making progress with leaders in other fields toward solutions that embrace diverse perspectives and the interconnectedness of all lifeforms and the planet.
Thousands of Vaccine Volunteers Got a Dummy Shot. Should They Get the Real Deal Now?
If a treatment or prevention is known to work, it is considered unethical to withhold it from volunteers in order to prolong a research trial, experts say.
The highly anticipated rollout of a COVID-19 vaccine poses ethical considerations: When will trial volunteers who got a placebo be vaccinated? And how will this affect the data in those trials?
It's an issue that vaccine manufacturers and study investigators are wrestling with as the Food and Drug Administration is expected to grant emergency use authorization this weekend to a vaccine developed by Pfizer and the German company BioNTech. Another vaccine, produced by Moderna, is nearing approval in the United States.
The most vulnerable—health care workers and nursing home residents—are deemed eligible to receive the initial limited supply in accordance with priority recommendations from the Centers for Disease Control and Prevention (CDC).
With health care workers constituting an estimated 20 percent of trial participants, this question also comes to the fore: "Is it now ethically imperative that we offer them the vaccine, those who have had placebo?" says William Schaffner, an infectious diseases physician at Vanderbilt University and an adviser to the CDC's immunization practices committee.
When a "gold-standard" measure becomes available, participants in the placebo group "would ordinarily be notified" of the strong public health recommendation to opt for immunization, says Johan Bester, interim assistant dean for biomedical science education and director of bioethics at the University of Nevada, Las Vegas School of Medicine.
"If a treatment or prevention exists that we know works, it is unethical to withhold it from people who would benefit from it just to answer a research question." This moral principle poses a quandary for ethicists and physicians alike, as they ponder possible paths to proceed with vaccination amid ongoing trials. Rigorous trials are double-blinded—neither the participants nor the investigators know who received the actual vaccine and who got a dummy injection.
"The intent of these trials is to follow these folks for up to two years," says Marci Drees, infection prevention officer and hospital epidemiologist for ChristianaCare in Wilmington, Delaware. At a minimum, she adds, researchers would prefer to monitor participants for six months.
"You can still follow safety over a long-term period of time without actually continuing to have a placebo group for comparison."
But in the midst of a pandemic, that may not be feasible. Prolonged exposure to the highly contagious and lethal virus could have dire consequences.
To avoid compromising the integrity of the blinded data, "there are some potentially creative solutions," Drees says. For instance, trial participants could receive the opposite of what they initially got, whether it was the vaccine or the placebo.
One factor in this decision-making process depends on when a particular trial is slated to conclude. If that time is approaching, the risk of waiting would be lower than if the trial is only halfway in progress, says Eric Lofgren, an epidemiologist at Washington State University who has studied the impact of COVID-19 in jails and at in-person sporting events.
Sometimes a study concludes earlier than the projected completion date. "All clinical trials have a data and safety monitoring board that reviews the interim results," Lofgren says. The board may halt a trial after finding evidence of harm, or when a treatment or vaccine has proven to be "sufficiently good," rendering it unethical to deprive the placebo group of its benefits.
The initial months of a trial are most crucial for assessing a vaccine's safety. Differences between the trial groups would be illuminating if fewer individuals who got the active vaccine contracted the virus and developed symptoms when compared to the placebo recipients. After that point, in vaccine-administered participants, "you can still follow safety over a long-term period of time without actually continuing to have a placebo group for comparison," says Dial Hewlett Jr., medical director for disease control at the Westchester County Department of Health in New York.
Even outside of a trial, safety is paramount and any severe side effects that occur will be closely monitored and investigated through national reporting networks. For example, regulators in the U.K. are investigating several rare but serious allergic reactions to the Pfizer vaccine given on Tuesday. The FDA has asked Pfizer to track allergic reactions in its safety monitoring plan, and some experts are proposing that Pfizer conduct a separate study of the vaccine on people with a history of severe allergies.
As the FDA eventually grants authorization to multiple vaccines, more participants are likely to leave trials and opt to be vaccinated. It is important that enough participants choose to stay in ongoing trials, says Nicole Hassoun, professor of philosophy at the State University of New York at Binghamton, where she directs the Global Health Impact program to extend medical access to the poor.
She's hopeful that younger participants and individuals without underlying medical conditions will make that determination. But the departure of too many participants at high risk for the virus would make it more difficult to evaluate the vaccine's safety and efficacy in those populations, Hassoun says, while acknowledging, "We can't have the best of both worlds."
Once a safe and effective vaccine is approved in the United States, "it would not be ethically appropriate to do placebo trials to test new vaccines."
One solution would entail allowing health care workers to exit a trial after a vaccine is approved, even though this would result in "a conundrum when the next group of people are brought forward to get the vaccine—whether they're people age 65 and older or they're essential workers, or whoever they are," says Vanderbilt physician Schaffner, who is a former board member of the Infectious Diseases Society of America. "All of a sudden, you'll have an erosion of the volunteers who are in the trial."
For now, one way or another, experts agree that current and subsequent trials should proceed. There is a compelling reason to identify additional vaccines with potentially greater effectiveness but with fewer side effects or less complex delivery methods that don't require storage at extremely low temperatures.
"Continuing with existing vaccine trials and starting others remains important," says Nir Eyal, professor and director of Rutgers University's Center for Population-Level Bioethics in New Brunswick, New Jersey. "We still need to tell how much proven vaccines block infections and how long their duration lasts. And populations around the world need vaccines that are easier to store and deliver, or simply cheaper."
But once a safe and effective vaccine is approved in the United States, "it would not be ethically appropriate to do placebo trials to test new vaccines," says bioethicist Bester at the University of Nevada, Las Vegas School of Medicine. "One possibility if a new vaccine emerges, is to test it against existing vaccines."
In a letter sent to trial volunteers in November, Pfizer and BioNTech committed to establishing "a process that would allow interested participants in the placebo group who meet the eligibility criteria for early access in their country to 'cross-over' to the vaccine group." The trial plans to continue monitoring all subjects regardless of whether people in the placebo group cross over, Pfizer said in a presentation to the FDA today. After Pfizer has collected six months of safety data, in April 2021, it plans to ask the FDA for full approval of the vaccine.
In the meantime, the company pledged to update volunteers as they obtain more input from regulatory authorities. "Thank you again for making a difference by being a part of this study," they wrote. "It is only through the efforts of volunteers like you that reaching this important milestone and developing a potential vaccine against COVID-19 is possible."
CORRECTION: An earlier version of this article mistakenly stated that the FDA would be granting emergency "approval" to the Pfizer/BioNTech vaccine, rather than "emergency use authorization." We regret the error.
Current research pipelines in biotech could take over a decade unless the heightened attention garners more resources, experts say.
Since March, 35 patients in the care of Dr. Gregory Jicha, a neurologist at the University of Kentucky, have died of Alzheimer's disease or related dementia.
Meanwhile, with 233 active clinical trials underway to find treatments, Jicha wonders why mainstream media outlets don't do more to highlight potential solutions to the physical, emotional and economic devastation of these diseases. "Unfortunately, it's not until we're right at the cusp of a major discovery that anybody pays attention to these very promising agents," he says.
Heightened awareness would bring more resources for faster progress, according to Jicha. Otherwise, he's concerned that current research pipelines will take over a decade.
In recent years, newspapers with national readerships have devoted more technology reporting to key developments in social media, artificial intelligence, wired gadgets and telecom. Less prominent has been news about biotech—innovations based on biology research—and new medicines emerging from this technology. That's the impression of Jicha as well as Craig Lipset, former head of clinical innovation at Pfizer. "Scientists and clinicians are entirely invested [in biotech], yet no one talks about their discoveries," he says.
With the popular press rightly focusing on progress with a vaccine for COVID-19 this year, notable developments in biomarkers, Alzheimer's and cancer research, gene therapies for cystic fibrosis, and therapeutics related to biological age may be going unreported. Jennifer Goldsack, Executive Director of the nonprofit Digital Medicine Society, is confused over the media's soft touch with biotech. "I'm genuinely interested in understanding what editors of technology sections think the public wants to be reading."
The Numbers on Media Coverage
A newspaper's health section is a sensible fit for biotech reporting. In 2020, these departments have concentrated largely on COVID-19—as they should—while sections on technology and science don't necessarily pick up on other biotech news. Emily Mullin, staff writer for the tech magazine OneZero, has observed a gap in newspaper coverage. "You have a lot of [niche outlets] reporting biotech on the business side for industry experts, and you have a lot of reporting heavily from the science side focused on [readers who are] scientists. But there aren't a lot of outlets doing more humanizing coverage of biotech."
Indeed, the volume of coverage by top-tier media outlets in the U.S. for non-COVID biotech has dropped 32 percent since the pandemic spiked in March, according to an analysis run for this article by Commetric, a company that looks at media reputation for clients in many sectors including biotech and artificial intelligence. Meanwhile, the volume of coverage for AI has held steady, up one percent.
Commetric's CEO, Magnus Hakansson, thinks important biotech stories were omitted from mainstream coverage even before the world fell into the grips of the virus. "Apart from COVID, it's been extremely difficult for biotech companies to push out their discoveries," he says. "People in biotech have to be quite creative when they want to communicate [progress in] different therapeutic areas, and that is a problem."
In mid-February, just before the pandemic dominated the news cycle, researchers used machine learning to find a powerful new antibiotic capable of killing strains of disease-causing bacteria that had previously resisted all known antibiotics. Science-focused outlets hailed the work as a breakthrough, but some nationally-read newspapers didn't mention it. "There is this very silent crisis around antibiotic resistance that no one is aware of," says Goldsack. "We could be 50 years away from not being able to give elective surgeries because we are at such a high risk of being unable to control infection."
Could mainstream media strike a better balance between cynicism toward biotech and hyping animal studies that probably won't ever benefit the humans reading about them?
What's to Gain from More Mainstream Biotech
A brighter public spotlight on biotech could result in greater support and faster progress with research, says Lipset. "One of the biggest delays in drug development is patient recruitment. Patients don't know about the opportunities," he said, because, "clinical research pipelines aren't talked about in the mainstream news." Only about eight percent of oncology patients participate.
The current focus on COVID-19, while warranted, could also be excluding lines of research that seem separate from the virus, but are actually relevant. In September, Nir Barzilai, director of the Institute of Aging Research at Albert Einstein College of Medicine, told me about eight different observational studies finding decreased COVID-19 severity among people taking a drug called metformin, which is believed to slow down the major hallmarks of biological aging, such as inflammation. Once a vaccine is approved and distributed, biologically older people could supplement it with metformin.
"Shining the spotlight on this research now could really be critical because COVID has shown what happens in older adults and how they're more at risk," says Jenna Bartley, a researcher of aging and immunology at the University of Connecticut, but she believes mainstream media sometimes miss stories on anti-aging therapies or portray them inaccurately.
The question remains why.
The Theranos Effect and Other Image Problems
Before the pandemic, Mullin, the biotech writer at OneZero, looked into a story for her editor about a company with a new test for infectious diseases. The company said its test, based on technology for editing genes, was fast, easy to use, and could be tailored to any pathogen. Mullin told her editor the evidence for the test's validity was impressive.
He wondered if readers would agree. "This is starting to sound like Theranos," he said.
The brainchild of entrepreneur Elizabeth Holmes, Theranos was valued at $9 billion in 2014. Time Magazine named Holmes one of its most influential people, and the blood-testing company was heavily covered by the media as a game changer for health outcomes—until Holmes was exposed by the Wall Street Journal as a fraud and criminally charged.
In the OneZero article, Mullin and her editor were careful to explain the gene-editing tech was legit, explicitly distinguishing it from Theranos. "I was like, yes—but this actually works! And they can show it works."
While the Holmes scandal explains some of the mistrust, it's part of a bigger pattern. The public's hopes for biotech have been frustrated repeatedly in recent decades, fostering a media mantra of fool me twice, shame on me. A recent report by Commetric noted that after the bursting of the biotech market bubble in the early 2000s, commentators grew deeply skeptical of the field. An additional source of caution may be the number of researchers in biotech with conflicts of interest such as patents or their own startups. "It's a landmine," Mullin said. "We're conditioned to think that scientists are out for the common good, but they have their own biases."
Yet another source of uncertainty: the long regulatory road and cost for new therapies to be approved by the FDA. The process can take 15 years and over a billion dollars; the percentage of drugs actually crossing the final strand of red tape is notoriously low.
"The only time stories have reached the news is when there's a sensational headline about the cure for cancer," said Lipset, "when, in fact it's about mice, and then things drop off." Meanwhile, consumer protection hurdles for some technologies, such as computer chips, are less onerous than the FDA gauntlet for new medicines. The media may view research breakthroughs in digital tech as more impactful because they're likelier to find their way into commercially available products.
And whereas a handful of digital innovations have been democratized for widespread consumption—96 percent of Americans now own a cell phone, and 72 percent use social media—journalists at nationally-read newspapers may see biotech as less attainable for the average reader. Sure, we're all aging, but will the healthcare system grant everyone fair access to treatments for slowing the aging process? Current disparities in healthcare sow reason for doubt.
And yet. Recall Lipset's point that more press coverage would drive greater participation in clinical trials, which could accelerate them and diversify participants. Could mainstream media strike a better balance between cynicism toward biotech and hyping animal studies that probably won't ever benefit the humans reading about them?
Biotech in a Post-COVID World
Imagine it's early 2022. Hopefully, much of the population is protected from the virus through some combination of vaccines, therapeutics, and herd immunity. We're starting to bounce back from the social and economic shocks of 2020. COVID-19 headlines recede from the front pages, then disappear altogether. Gradually, certain aspects of life pick up where they left off in 2019, while a few changes forced by the pandemic prove to be more lasting, some for the better.
Among its possible legacies, the virus could usher in a new era of biotech development and press coverage, with these two trends reinforcing each other. While government has mismanaged its response to the virus, the level of innovation, collaboration and investment in pandemic-related biotech has been compared to the Manhattan Project. "There's no question that vaccine acceleration is a success story," said Kevin Schulman, a professor of medicine and economics at Stanford. "We could use this experience to build new economic models to correct market failures. It could carry over to oncology or Alzheimer's."
As Winston Churchill said, never let a good crisis go to waste.
Lipset thinks the virus has primed us to pay attention, bringing biotech into the public's consciousness like never before. He's amazed at how many neighbors and old friends from high school are coming out of the woodwork to ask him how clinical trials work. "What happens next is interesting. Does this open a window of opportunity to get more content out? People's appetites have been whetted."
High-profile wins could help to sustain interest, such as deployment of rapid tests of COVID-19 to be taken at home, a version of which the FDA authorized on November 18th. The idea bears resemblance to the Theranos concept, also designed as a portable analysis, except this test met the FDA's requirements and has a legitimate chance of changing people's lives. Meanwhile, at least two vaccines are on track to gain government approval in record time. The unprecedented speed could be a catalyst for streamlining inefficiencies in the FDA's approval process in non-emergency situations.
Tests for COVID-19 represent what some view as the future of managing diseases: early detection. This paradigm may be more feasible—and deserving of journalistic ink—than research on diseases in advanced stages, says Azra Raza, professor of medicine at Columbia University. "Journalists have to challenge this conceit of thinking we can cure end-stage cancer," says Raza, author of The First Cell. Beyond animal studies and "exercise helps" articles, she thinks writers should focus on biotech for catching the earliest footprints of cancer when it's more treatable. "Not enough people appreciate the extent of this tragedy, but journalists can help us do it. COVID-19 is a great moment of truth telling."
Another pressing truth is the need for vaccination, as half of Americans have said they'll skip them due to concerns about safety and effectiveness. It's not the kind of stumbling block faced by iPhones or social media algorithms. AI stirs plenty of its own controversy, but the public's interest in learning about AI and engaging with it seems to grow regardless. "Who are the publicists doing such a good job for AI that biotechnology is lacking?" Lipset wonders.
The job description of those publicists, whoever they are, could be expanding. Scientists are increasingly using AI to measure the effects of new medicines that target diseases—including COVID-19—and the pathways of aging. Mullin noted the challenge of reporting breakthroughs in the life sciences in ways the public understands. With many newsrooms tightening budgets, fewer writers have science backgrounds, and "biotech is daunting for journalists," she says. "It's daunting for me and I work in this area." Now factor in the additional expertise required to understand biotech and AI. "I learned the ropes for how to read a biotech paper, but I have no idea how to read an AI paper."
Nevertheless, Mullin believes reporters have a duty to scrutinize whether this convergence of AI and biotech will foster better outcomes. "Is it just the shiny new tool we're employing because we can? Will algorithms help eliminate health disparities or contribute to them even more? We need to pay attention."

