New tools could catch disease outbreaks earlier - or predict them

A team at the Catalan Institution for Research and Advanced Studies is utilizing drones and weather stations to collect data on how mosquito breeding patterns are changing in response to climate shifts.
Every year, the villages which lie in the so-called ‘Nipah belt’— which stretches along the western border between Bangladesh and India, brace themselves for the latest outbreak. For since 1998, when Nipah virus—a form of hemorrhagic fever most common in Bangladesh—first spilled over into humans, it has been a grim annual visitor to the people of this region.
With a 70 percent fatality rate, no vaccine, and no known treatments, Nipah virus has been dubbed in the Western world as ‘the worst disease no one has ever heard of.’ Currently, outbreaks tend to be relatively contained because it is not very transmissible. The virus circulates throughout Asia in fruit eating bats, and only tends to be passed on to people who consume contaminated date palm sap, a sweet drink which is harvested across Bangladesh.
But as SARS-CoV-2 has shown the world, this can quickly change.
“Nipah virus is among what virologists call ‘the Big 10,’ along with things like Lassa fever and Crimean Congo hemorrhagic fever,” says Noam Ross, a disease ecologist at New York-based non-profit EcoHealth Alliance. “These are pretty dangerous viruses from a lethality perspective, which don’t currently have the capacity to spread into broader human populations. But that can evolve, and you could very well see a variant emerge that has human-human transmission capability.”
That’s not an overstatement. Surveys suggest that mammals harbour about 40,000 viruses, with roughly a quarter capable of infecting humans. The vast majority never get a chance to do so because we don’t encounter them, but climate change can alter that. Recent studies have found that as animals relocate to new habitats due to shifting environmental conditions, the coming decades will bring around 300,000 first encounters between species which normally don’t interact, especially in tropical Africa and southeast Asia. All these interactions will make it far more likely for hitherto unknown viruses to cross paths with humans.
That’s why for the last 16 years, EcoHealth Alliance has been conducting ongoing viral surveillance projects across Bangladesh. The goal is to understand why Nipah is so much more prevalent in the western part of the country, compared to the east, and keep a watchful eye out for new Nipah strains as well as other dangerous pathogens like Ebola.
"There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them," says Cat Lippi, medical geography researcher at the University of Florida.
Until very recently this kind of work has been hampered by the limitations of viral surveillance technology. The PREDICT project, a $200 million initiative funded by the United States Agency for International Development, which conducted surveillance across the Amazon Basin, Congo Basin and extensive parts of South and Southeast Asia, relied upon so-called nucleic acid assays which enabled scientists to search for the genetic material of viruses in animal samples.
However, the project came under criticism for being highly inefficient. “That approach requires a big sampling effort, because of the rarity of individual infections,” says Ross. “Any particular animal may be infected for a couple of weeks, maybe once or twice in its lifetime. So if you sample thousands and thousands of animals, you'll eventually get one that has an Ebola virus infection right now.”
Ross explains that there is now far more interest in serological sampling—the scientific term for the process of drawing blood for antibody testing. By searching for the presence of antibodies in the blood of humans and animals, scientists have a greater chance of detecting viruses which started circulating recently.
Despite the controversy surrounding EcoHealth Alliance’s involvement in so-called gain of function research—experiments that study whether viruses might mutate into deadlier strains—the organization’s separate efforts to stay one step ahead of pathogen evolution are key to stopping the next pandemic.
“Having really cheap and fast surveillance is really important,” says Ross. “Particularly in a place where there's persistent, low level, moderate infections that potentially have the ability to develop into more epidemic or pandemic situations. It means there’s a pathway that something more dangerous can come through."
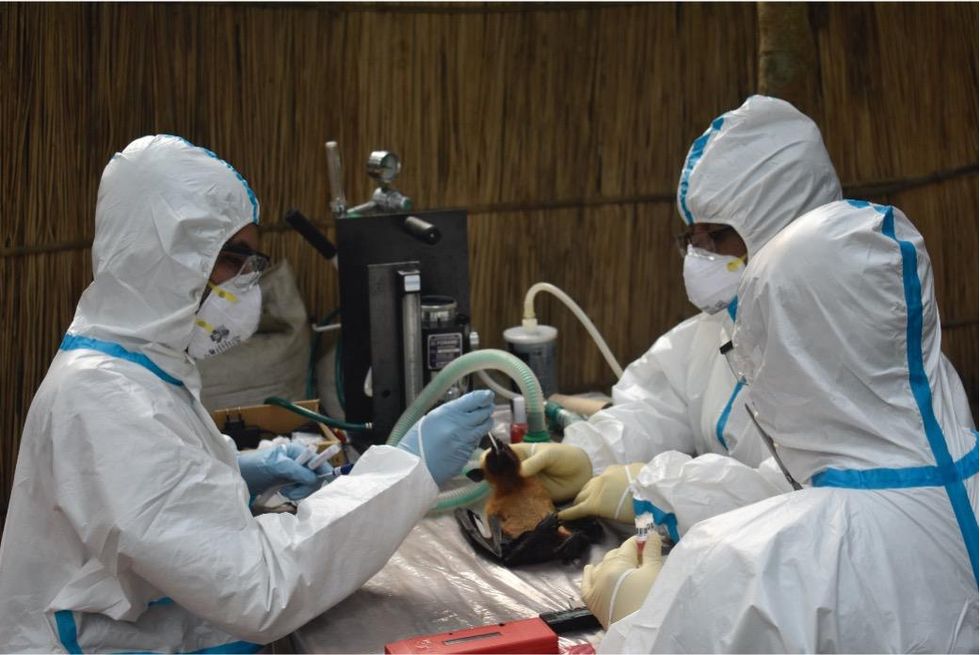
Scientists are searching for the presence of antibodies in the blood of humans and animals in hopes to detect viruses that recently started circulating.
EcoHealth Alliance
In Bangladesh, EcoHealth Alliance is attempting to do this using a newer serological technology known as a multiplex Luminex assay, which tests samples against a panel of known antibodies against many different viruses. It collects what Ross describes as a ‘footprint of information,’ which allows scientists to tell whether the sample contains the presence of a known pathogen or something completely different and needs to be investigated further.
By using this technology to sample human and animal populations across the country, they hope to gain an idea of whether there are any novel Nipah virus variants or strains from the same family, as well as other deadly viral families like Ebola.
This is just one of several novel tools being used for viral discovery in surveillance projects around the globe. Multiple research groups are taking PREDICT’s approach of looking for novel viruses in animals in various hotspots. They collect environmental DNA—mucus, faeces or shed skin left behind in soil, sediment or water—which can then be genetically sequenced.
Five years ago, this would have been a painstaking work requiring bringing collected samples back to labs. Today, thanks to the vast amounts of money spent on new technologies during COVID-19, researchers now have portable sequencing tools they can take out into the field.
Christopher Jerde, a researcher at the UC Santa Barbara Marine Science Institute, points to the Oxford Nanopore MinION sequencer as one example. “I tried one of the early versions of it four years ago, and it was miserable,” he says. “But they’ve really improved, and what we’re going to be able to do in the next five to ten years will be amazing. Instead of having to carefully transport samples back to the lab, we're going to have cigar box-shaped sequencers that we take into the field, plug into a laptop, and do the whole sequencing of an organism.”
In the past, viral surveillance has had to be very targeted and focused on known families of viruses, potentially missing new, previously unknown zoonotic pathogens. Jerde says that the rise of portable sequencers will lead to what he describes as “true surveillance.”
“Before, this was just too complex,” he says. “It had to be very focused, for example, looking for SARS-type viruses. Now we’re able to say, ‘Tell us all the viruses that are here?’ And this will give us true surveillance – we’ll be able to see the diversity of all the pathogens which are in these spots and have an understanding of which ones are coming into the population and causing damage.”
But being able to discover more viruses also comes with certain challenges. Some scientists fear that the speed of viral discovery will soon outpace the human capacity to analyze them all and assess the threat that they pose to us.
“I think we're already there,” says Jason Ladner, assistant professor at Northern Arizona University’s Pathogen and Microbiome Institute. “If you look at all the papers on the expanding RNA virus sphere, there are all of these deposited partial or complete viral sequences in groups that we just don't know anything really about yet.” Bats, for example, carry a myriad of viruses, whose ability to infect human cells we understand very poorly.
Cultivating these viruses under laboratory conditions and testing them on organoids— miniature, simplified versions of organs created from stem cells—can help with these assessments, but it is a slow and painstaking work. One hope is that in the future, machine learning could help automate this process. The new SpillOver Viral Risk Ranking platform aims to assess the risk level of a given virus based on 31 different metrics, while other computer models have tried to do the same based on the similarity of a virus’s genomic sequence to known zoonotic threats.
However, Ladner says that these types of comparisons are still overly simplistic. For one thing, scientists are still only aware of a few hundred zoonotic viruses, which is a very limited data sample for accurately assessing a novel pathogen. Instead, he says that there is a need for virologists to develop models which can determine viral compatibility with human cells, based on genomic data.
“One thing which is really useful, but can be challenging to do, is understand the cell surface receptors that a given virus might use,” he says. “Understanding whether a virus is likely to be able to use proteins on the surface of human cells to gain entry can be very informative.”
As the Earth’s climate heats up, scientists also need to better model the so-called vector borne diseases such as dengue, Zika, chikungunya and yellow fever. Transmitted by the Aedes mosquito residing in humid climates, these blights currently disproportionally affect people in low-income nations. But predictions suggest that as the planet warms and the pests find new homes, an estimated one billion people who currently don’t encounter them might be threatened by their bites by 2080. “When it comes to mosquito-borne diseases we have to worry about shifts in suitable habitat,” says Cat Lippi, a medical geography researcher at the University of Florida. “As climate patterns change on these big scales, we expect to see shifts in where people will be at risk for contracting these diseases.”
Public health practitioners and government decision-makers need tools to make climate-informed decisions about the evolving threat of different infectious diseases. Some projects are already underway. An ongoing collaboration between the Catalan Institution for Research and Advanced Studies and researchers in Brazil and Peru is utilizing drones and weather stations to collect data on how mosquitoes change their breeding patterns in response to climate shifts. This information will then be fed into computer algorithms to predict the impact of mosquito-borne illnesses on different regions.

The team at the Catalan Institution for Research and Advanced Studies is using drones and weather stations to collect data on how mosquito breeding patterns change due to climate shifts.
Gabriel Carrasco
Lippi says that similar models are urgently needed to predict how changing climate patterns affect respiratory, foodborne, waterborne and soilborne illnesses. The UK-based Wellcome Trust has allocated significant assets to fund such projects, which should allow scientists to monitor the impact of climate on a much broader range of infections. “There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them,” she says.
COVID-19’s havoc boosted funding for infectious disease research, but as its threats begin to fade from policymakers’ focus, the money may dry up. Meanwhile, scientists warn that another major infectious disease outbreak is inevitable, potentially within the next decade, so combing the planet for pathogens is vital. “Surveillance is ultimately a really boring thing that a lot of people don't want to put money into, until we have a wide scale pandemic,” Jerde says, but that vigilance is key to thwarting the next deadly horror. “It takes a lot of patience and perseverance to keep looking.”
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are increasing vulnerabilities to infectious diseases by land and by sea. The magazine probes how scientists are making progress with leaders in other fields toward solutions that embrace diverse perspectives and the interconnectedness of all lifeforms and the planet.
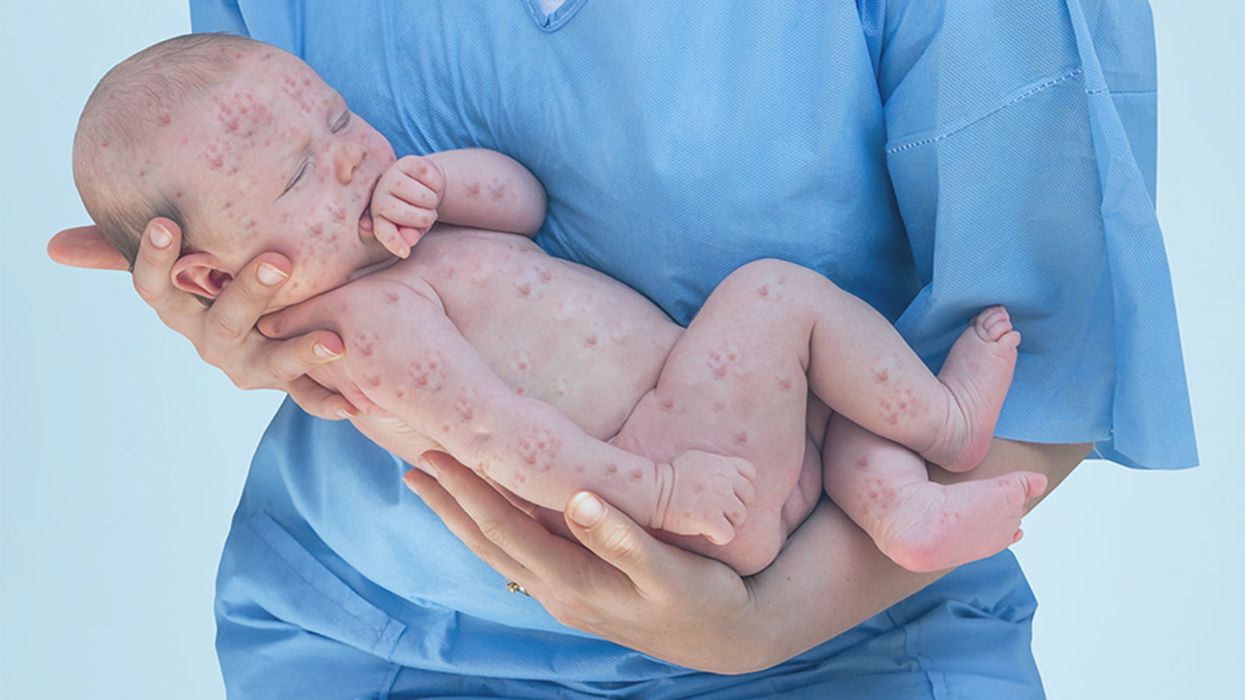
A doctor cradles a newborn who is sick with measles.
Ethan Lindenberger, the Ohio teenager who sought out vaccinations after he was denied them as a child, recently testified before Congress about why his parents became anti-vaxxers. The trouble, he believes, stems from the pervasiveness of misinformation online.
There is evidence that 'educating' people with facts about the benefits of vaccination may not be effective.
"For my mother, her love and affection and care as a parent was used to push an agenda to create a false distress," he told the Senate Committee. His mother read posts on social media saying vaccines are dangerous, and that was enough to persuade her against them.
His story is an example of how widespread and harmful the current discourse on vaccinations is—and more importantly—how traditional strategies to convince people about the merits of vaccination have largely failed.
As responsible members of society, all of us have implicitly signed on to what ethicists call the "Social Contract" -- we agree to abide by certain moral and political rules of behavior. This is what our societal values, norms, and often governments are based upon. However, with the unprecedented rise of social media, alternative facts, and fake news, it is evident that our understanding—and application—of the social contract must also evolve.
Nowhere is this breakdown of societal norms more visible than in the failure to contain the spread of vaccine-preventable diseases like measles. What started off as unexplained episodes in New York City last October, mostly in communities that are under-vaccinated, has exploded into a national epidemic: 880 cases of measles across 24 states in 2019, according to the CDC (as of May 17, 2019). In fact, the Unites States is only eight months away from losing its "measles free" status, joining Venezuela as the second country out of North and South America with that status.
The U.S. is not the only country facing this growing problem. Such constant and perilous reemergence of measles and other vaccine-preventable diseases in various parts of the world raises doubts about the efficacy of current vaccination policies. In addition to the loss of valuable life, these outbreaks lead to loss of millions of dollars in unnecessary expenditure of scarce healthcare resources. While we may be living through an age of information, we are also navigating an era whose hallmark is a massive onslaught on truth.
There is ample evidence on how these outbreaks start: low-vaccination rates. At the same time, there is evidence that 'educating' people with facts about the benefits of vaccination may not be effective. Indeed, human reasoning has a limit, and facts alone rarely change a person's opinion. In a fascinating report by researchers from the University of Pennsylvania, a small experiment revealed how "behavioral nudges" could inform policy decisions around vaccination.
In the reported experiment, the vaccination rate for employees of a company increased by 1.5 percent when they were prompted to name the date when they planned to get their flu shot. In the same experiment, when employees were prompted to name both a date and a time for their planned flu shot, vaccination rate increased by 4 percent.
A randomized trial revealed the subtle power of "announcements" – direct, brief, assertive statements by physicians that assumed parents were ready to vaccinate their children.
This experiment is a part of an emerging field of behavioral economics—a scientific undertaking that uses insights from psychology to understand human decision-making. The field was born from a humbling realization that humans probably do not possess an unlimited capacity for processing information. Work in this field could inform how we can formulate vaccination policy that is effective, conserves healthcare resources, and is applicable to current societal norms.
Take, for instance, the case of Human Papilloma Virus (HPV) that can cause several types of cancers in both men and women. Research into the quality of physician communication has repeatedly revealed how lukewarm recommendations for HPV vaccination by primary care physicians likely contributes to under-immunization of eligible adolescents and can cause confusion for parents.
A randomized trial revealed the subtle power of "announcements" – direct, brief, assertive statements by physicians that assumed parents were ready to vaccinate their children. These announcements increased vaccination rates by 5.4 percent. Lengthy, open-ended dialogues demonstrated no benefit in vaccination rates. It seems that uncertainty from the physician translates to unwillingness from a parent.
Choice architecture is another compelling concept. The premise is simple: We hardly make any of our decisions in vacuum; the environment in which these decisions are made has an influence. If health systems were designed with these insights in mind, people would be more likely to make better choices—without being forced.
This theory, proposed by Richard Thaler, who won the 2017 Nobel Prize in Economics, was put to the test by physicians at the University of Pennsylvania. In their study, flu vaccination rates at primary care practices increased by 9.5 percent all because the staff implemented "active choice intervention" in their electronic health records—a prompt that nudged doctors and nurses to ask patients if they'd gotten the vaccine yet. This study illustrated how an intervention as simple as a reminder can save lives.
To be sure, some bioethicists do worry about implementing these policies. Are behavioral nudges akin to increased scrutiny or a burden for the disadvantaged? For example, would incentives to quit smoking unfairly target the poor, who are more likely to receive criticism for bad choices?
The measles outbreak is a sober reminder of how devastating it can be when the social contract breaks down.
While this is a valid concern, behavioral economics offers one of the only ethical solutions to increasing vaccination rates by addressing the most critical—and often legal—challenge to universal vaccinations: mandates. Choice architecture and other interventions encourage and inform a choice, allowing an individual to retain his or her right to refuse unwanted treatment. This distinction is especially important, as evidence suggests that people who refuse vaccinations often do so as a result of cognitive biases – systematic errors in thinking resulting from emotional attachment or a lack of information.
For instance, people are prone to "confirmation bias," or a tendency to selectively believe in information that confirms their preexisting theories, rather than the available evidence. At the same time, people do not like mandates. In such situations, choice architecture provides a useful option: people are nudged to make the right choice via the design of health delivery systems, without needing policies that rely on force.
The measles outbreak is a sober reminder of how devastating it can be when the social contract breaks down and people fall prey to misinformation. But all is not lost. As we fight a larger societal battle against alternative facts, we now have another option in the trenches to subtly encourage people to make better choices.
Using insights from research in decision-making, we can all contribute meaningfully in controversial conversations with family, friends, neighbors, colleagues, and our representatives — and push for policies that protect those we care about. A little more than a hundred years ago, thousands of lives were routinely lost to preventive illnesses. We've come too far to let ignorance destroy us now.
New Tech Can Predict Breast Cancer Years in Advance
Breast cancer survivors rally in a support group.
Every two minutes, a woman is diagnosed with breast cancer. The question is, can those at high risk be identified early enough to survive?
New AI software has predicted risk equally well in both white and black women for the first time.
The current standard practice in medicine is not exactly precise. It relies on age, family history of cancer, and breast density, among other factors, to determine risk. But these factors do not always tell the whole story, leaving many women to slip through the cracks. In addition, a racial gap persists in breast cancer treatment and survival. African-American women are 42 percent more likely to die from the disease despite relatively equal rates of diagnosis.
But now those grim statistics could be changing. A team of researchers from MIT's Computer Science and Artificial Intelligence Laboratory have developed a deep learning model that can more accurately predict a patient's breast cancer risk compared to established clinical guidelines – and it has predicted risk equally well in both white and black women for the first time.
The Lowdown
Study results published in Radiology described how the AI software read mammogram images from more than 60,000 patients at Massachusetts General Hospital to identify subtle differences in breast tissue that pointed to potential risk factors, even in their earliest stages. The team accessed the patients' actual diagnoses and determined that the AI model was able to correctly place 31 percent of all cancer patients in the highest-risk category of developing breast cancer within five years of the examination, compared to just 18 percent for existing models.
"Each image has hundreds of thousands of pixels identifying something that may not necessarily be detected by the human eye," said MIT professor Regina Barzilay, one of the study's lead authors. "We all have limited visual capacities so it seems some machines trained on hundreds of thousands of images with a known outcome can capture correlations the human eye might not notice."
Barzilay, a breast cancer survivor herself, had abnormal tissue patterns on mammograms in 2012 and 2013, but wasn't diagnosed until after a 2014 image reading, illustrating the limitations of human processing alone.

MIT professor Regina Barzilay, a lead author on the new study and a breast cancer survivor herself.
(Courtesy MIT)
Next up: The MIT team is looking at training the model to detect other cancers and health risks. Barzilay recalls how a cardiologist told her during a conference that women with heart diseases had a different pattern of calcification on their mammograms, demonstrating how already existing images can be used to extract other pieces of information about a person's health status.
Integration of the AI model in standard care could help doctors better tailor screening and prevention programs based on actual instead of perceived risk. Patients who might register as higher risk by current guidelines could be identified as lower risk, helping resolve conflicting opinions about how early and how often women should receive mammograms.
Open Questions: While the results were promising, it's unknown how well the model will work on a larger scale, as the study looked at data from just one institution and used mammograms supplied by just one hospital. Some risk factor information was also unavailable for certain patients during the study, leaving researchers unable to fully compare the AI model's performance to that of the traditional standard.
One incentive to wider implementation and study, however, is the bonus that no new hardware is required to use the AI model. With other institutions now showing interest, this software could lead to earlier routine detection and treatment of breast cancer — resulting in more lives saved.