New tools could catch disease outbreaks earlier - or predict them

A team at the Catalan Institution for Research and Advanced Studies is utilizing drones and weather stations to collect data on how mosquito breeding patterns are changing in response to climate shifts.
Every year, the villages which lie in the so-called ‘Nipah belt’— which stretches along the western border between Bangladesh and India, brace themselves for the latest outbreak. For since 1998, when Nipah virus—a form of hemorrhagic fever most common in Bangladesh—first spilled over into humans, it has been a grim annual visitor to the people of this region.
With a 70 percent fatality rate, no vaccine, and no known treatments, Nipah virus has been dubbed in the Western world as ‘the worst disease no one has ever heard of.’ Currently, outbreaks tend to be relatively contained because it is not very transmissible. The virus circulates throughout Asia in fruit eating bats, and only tends to be passed on to people who consume contaminated date palm sap, a sweet drink which is harvested across Bangladesh.
But as SARS-CoV-2 has shown the world, this can quickly change.
“Nipah virus is among what virologists call ‘the Big 10,’ along with things like Lassa fever and Crimean Congo hemorrhagic fever,” says Noam Ross, a disease ecologist at New York-based non-profit EcoHealth Alliance. “These are pretty dangerous viruses from a lethality perspective, which don’t currently have the capacity to spread into broader human populations. But that can evolve, and you could very well see a variant emerge that has human-human transmission capability.”
That’s not an overstatement. Surveys suggest that mammals harbour about 40,000 viruses, with roughly a quarter capable of infecting humans. The vast majority never get a chance to do so because we don’t encounter them, but climate change can alter that. Recent studies have found that as animals relocate to new habitats due to shifting environmental conditions, the coming decades will bring around 300,000 first encounters between species which normally don’t interact, especially in tropical Africa and southeast Asia. All these interactions will make it far more likely for hitherto unknown viruses to cross paths with humans.
That’s why for the last 16 years, EcoHealth Alliance has been conducting ongoing viral surveillance projects across Bangladesh. The goal is to understand why Nipah is so much more prevalent in the western part of the country, compared to the east, and keep a watchful eye out for new Nipah strains as well as other dangerous pathogens like Ebola.
"There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them," says Cat Lippi, medical geography researcher at the University of Florida.
Until very recently this kind of work has been hampered by the limitations of viral surveillance technology. The PREDICT project, a $200 million initiative funded by the United States Agency for International Development, which conducted surveillance across the Amazon Basin, Congo Basin and extensive parts of South and Southeast Asia, relied upon so-called nucleic acid assays which enabled scientists to search for the genetic material of viruses in animal samples.
However, the project came under criticism for being highly inefficient. “That approach requires a big sampling effort, because of the rarity of individual infections,” says Ross. “Any particular animal may be infected for a couple of weeks, maybe once or twice in its lifetime. So if you sample thousands and thousands of animals, you'll eventually get one that has an Ebola virus infection right now.”
Ross explains that there is now far more interest in serological sampling—the scientific term for the process of drawing blood for antibody testing. By searching for the presence of antibodies in the blood of humans and animals, scientists have a greater chance of detecting viruses which started circulating recently.
Despite the controversy surrounding EcoHealth Alliance’s involvement in so-called gain of function research—experiments that study whether viruses might mutate into deadlier strains—the organization’s separate efforts to stay one step ahead of pathogen evolution are key to stopping the next pandemic.
“Having really cheap and fast surveillance is really important,” says Ross. “Particularly in a place where there's persistent, low level, moderate infections that potentially have the ability to develop into more epidemic or pandemic situations. It means there’s a pathway that something more dangerous can come through."
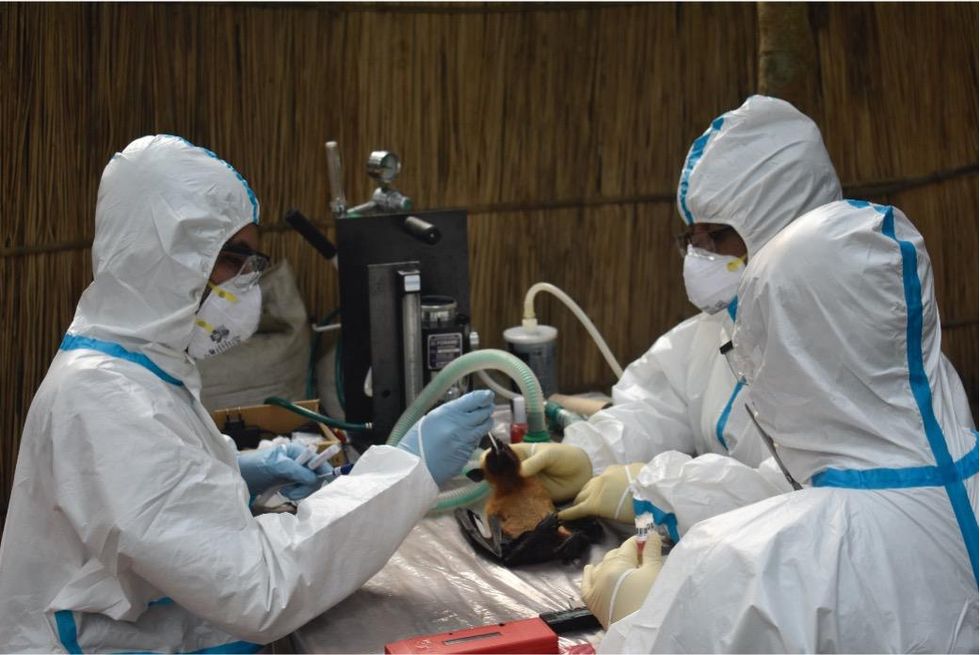
Scientists are searching for the presence of antibodies in the blood of humans and animals in hopes to detect viruses that recently started circulating.
EcoHealth Alliance
In Bangladesh, EcoHealth Alliance is attempting to do this using a newer serological technology known as a multiplex Luminex assay, which tests samples against a panel of known antibodies against many different viruses. It collects what Ross describes as a ‘footprint of information,’ which allows scientists to tell whether the sample contains the presence of a known pathogen or something completely different and needs to be investigated further.
By using this technology to sample human and animal populations across the country, they hope to gain an idea of whether there are any novel Nipah virus variants or strains from the same family, as well as other deadly viral families like Ebola.
This is just one of several novel tools being used for viral discovery in surveillance projects around the globe. Multiple research groups are taking PREDICT’s approach of looking for novel viruses in animals in various hotspots. They collect environmental DNA—mucus, faeces or shed skin left behind in soil, sediment or water—which can then be genetically sequenced.
Five years ago, this would have been a painstaking work requiring bringing collected samples back to labs. Today, thanks to the vast amounts of money spent on new technologies during COVID-19, researchers now have portable sequencing tools they can take out into the field.
Christopher Jerde, a researcher at the UC Santa Barbara Marine Science Institute, points to the Oxford Nanopore MinION sequencer as one example. “I tried one of the early versions of it four years ago, and it was miserable,” he says. “But they’ve really improved, and what we’re going to be able to do in the next five to ten years will be amazing. Instead of having to carefully transport samples back to the lab, we're going to have cigar box-shaped sequencers that we take into the field, plug into a laptop, and do the whole sequencing of an organism.”
In the past, viral surveillance has had to be very targeted and focused on known families of viruses, potentially missing new, previously unknown zoonotic pathogens. Jerde says that the rise of portable sequencers will lead to what he describes as “true surveillance.”
“Before, this was just too complex,” he says. “It had to be very focused, for example, looking for SARS-type viruses. Now we’re able to say, ‘Tell us all the viruses that are here?’ And this will give us true surveillance – we’ll be able to see the diversity of all the pathogens which are in these spots and have an understanding of which ones are coming into the population and causing damage.”
But being able to discover more viruses also comes with certain challenges. Some scientists fear that the speed of viral discovery will soon outpace the human capacity to analyze them all and assess the threat that they pose to us.
“I think we're already there,” says Jason Ladner, assistant professor at Northern Arizona University’s Pathogen and Microbiome Institute. “If you look at all the papers on the expanding RNA virus sphere, there are all of these deposited partial or complete viral sequences in groups that we just don't know anything really about yet.” Bats, for example, carry a myriad of viruses, whose ability to infect human cells we understand very poorly.
Cultivating these viruses under laboratory conditions and testing them on organoids— miniature, simplified versions of organs created from stem cells—can help with these assessments, but it is a slow and painstaking work. One hope is that in the future, machine learning could help automate this process. The new SpillOver Viral Risk Ranking platform aims to assess the risk level of a given virus based on 31 different metrics, while other computer models have tried to do the same based on the similarity of a virus’s genomic sequence to known zoonotic threats.
However, Ladner says that these types of comparisons are still overly simplistic. For one thing, scientists are still only aware of a few hundred zoonotic viruses, which is a very limited data sample for accurately assessing a novel pathogen. Instead, he says that there is a need for virologists to develop models which can determine viral compatibility with human cells, based on genomic data.
“One thing which is really useful, but can be challenging to do, is understand the cell surface receptors that a given virus might use,” he says. “Understanding whether a virus is likely to be able to use proteins on the surface of human cells to gain entry can be very informative.”
As the Earth’s climate heats up, scientists also need to better model the so-called vector borne diseases such as dengue, Zika, chikungunya and yellow fever. Transmitted by the Aedes mosquito residing in humid climates, these blights currently disproportionally affect people in low-income nations. But predictions suggest that as the planet warms and the pests find new homes, an estimated one billion people who currently don’t encounter them might be threatened by their bites by 2080. “When it comes to mosquito-borne diseases we have to worry about shifts in suitable habitat,” says Cat Lippi, a medical geography researcher at the University of Florida. “As climate patterns change on these big scales, we expect to see shifts in where people will be at risk for contracting these diseases.”
Public health practitioners and government decision-makers need tools to make climate-informed decisions about the evolving threat of different infectious diseases. Some projects are already underway. An ongoing collaboration between the Catalan Institution for Research and Advanced Studies and researchers in Brazil and Peru is utilizing drones and weather stations to collect data on how mosquitoes change their breeding patterns in response to climate shifts. This information will then be fed into computer algorithms to predict the impact of mosquito-borne illnesses on different regions.

The team at the Catalan Institution for Research and Advanced Studies is using drones and weather stations to collect data on how mosquito breeding patterns change due to climate shifts.
Gabriel Carrasco
Lippi says that similar models are urgently needed to predict how changing climate patterns affect respiratory, foodborne, waterborne and soilborne illnesses. The UK-based Wellcome Trust has allocated significant assets to fund such projects, which should allow scientists to monitor the impact of climate on a much broader range of infections. “There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them,” she says.
COVID-19’s havoc boosted funding for infectious disease research, but as its threats begin to fade from policymakers’ focus, the money may dry up. Meanwhile, scientists warn that another major infectious disease outbreak is inevitable, potentially within the next decade, so combing the planet for pathogens is vital. “Surveillance is ultimately a really boring thing that a lot of people don't want to put money into, until we have a wide scale pandemic,” Jerde says, but that vigilance is key to thwarting the next deadly horror. “It takes a lot of patience and perseverance to keep looking.”
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are increasing vulnerabilities to infectious diseases by land and by sea. The magazine probes how scientists are making progress with leaders in other fields toward solutions that embrace diverse perspectives and the interconnectedness of all lifeforms and the planet.
Not Vaccinating Your Kids Endangers Public Health
A pediatrician gives a one-year-old child a vaccine.
[Editor's Note: This opinion essay is in response to our current Big Question, which we posed to experts with different viewpoints: "Where should society draw the line between requiring vaccinations for children and allowing parental freedom of choice?"]
Society has a right and at times an obligation to require children to be vaccinated. Vaccines are one of the most effective medical and public health interventions. They save lives and prevent suffering. The vast majority of parents in the United States fully vaccinate their children according to the recommended immunization schedule. These parents are making decisions so that the interests of their children and the interest of society are the same. There are no ethical tensions.
"Measles is only a plane ride away from American children."
A strong scientific basis supports the recommended immunization schedule. The benefits of recommended vaccines are much bigger than the risks. However, a very small proportion of parents are ideologically opposed to vaccines. A slightly larger minority of parents do not believe that all of the recommended vaccines are in their child's best interests.
Forgoing vaccinations creates risk to the child of contracting diseases. It also creates risk to communities and vulnerable groups of people who cannot be vaccinated because of their age or health status.
For example, many vaccines are not able to be given to newborns, such as the measles vaccine which is recommended at 12-15 months of age, leaving young children vulnerable. Many diseases are particularly dangerous for young children. There are also some children who can't be vaccinated, such as pediatric cancer patients who are undergoing chemotherapy or radiation treatment. These children are at increased risk of serous complication or death.
Then there are people who are vaccinated but remain susceptible to disease because no vaccine is 100% effective. In the case of measles, two doses of vaccines protect 97% of people, leaving 3% still susceptible even after being fully vaccinated. All of these groups of people – too young, not eligible, and vaccinated but still susceptible – are dependent on almost everyone else to get vaccinated in order for them to be protected.
Sadly, even though measles has been largely controlled because most people get the very safe and very effective vaccine, we are now seeing dangerous new outbreaks because some parents are refusing vaccines for their children, especially in Europe. Children have died. Measles is only a plane ride away from American children.
There have been repeated measles outbreaks in the United States – such as the Disneyland outbreak and six outbreaks already this year - because of communities where too many parents refuse the vaccine and measles is brought over, often from Europe.
The public health benefits cannot be emphasized enough: Vaccines are not just about protecting your child. Vaccines protect other children and the entire community. Vaccine-preventable diseases (with the exception of tetanus) are spread from person to person. The decision of a parent to not vaccinate their child can endanger other children and vulnerable people.
As a vaccine safety researcher for 20 years, I believe that the community benefit of vaccination can provide justification to limit parental autonomy.
Given these tensions between parental autonomy and the protective value of vaccines, the fundamental question remains: Should society require all children to submit to vaccinations? As a vaccine safety researcher for 20 years, I believe that the community benefit of vaccination can provide justification to limit parental autonomy.
In the United States, we see this balancing act though state requirements for vaccinations to enter school and the varying availability of non-medical exemptions to these laws. Mandatory vaccination in the United States are all state laws. All states require children entering school to receive vaccines and permit medical exemptions. There are a lot of differences between states regarding which vaccines are required, target populations (daycare, school entry, middle school, college), and existence and types of non-medical (religious or philosophical) exemptions that are permitted.
Amid recent measles outbreaks, for instance, California eliminated all non-medical exemptions, making it one of three states that only permit medical exemptions. The existence and enforcement of these school laws reflect broad public support for vaccines to protect the community from disease outbreaks.
I worry about how many kids must suffer, and even die, from diseases like measles until enough is enough. Such tragedies have no place in the modern era. All parents want to do right by their children. All parents deserve autonomy when it comes to decision-making. But when their choices confer serious risks to others, the buck should stop. Our nation would be better off—both medically and ethically—if we did not turn our backs on our most vulnerable individuals.
[Editor's Note: Read the opposite viewpoint here.]
Your Body Has This Astonishing Magical Power
A fierce champion fighter in action, representing the incredible power of the human immune system.
It's vacation time. You and your family visit a country where you've never been and, in fact, your parents or grandparents had never been. You find yourself hiking beside a beautiful lake. It's a gorgeous day. You dive in. You are not alone.
How can your T cells and B cells react to a pathogen they've never seen?
In the water swim parasites, perhaps a parasite called giardia. The invader slips in through your mouth or your urinary tract. This bug is entirely new to you, and there's more. It might be new to everyone you've ever met or come into contact with. The parasite may have evolved in this setting for hundreds of thousands of years so that it's different from any giardia bug you've ever come into contact with before or that thrives in the region where you live.
How can your T cells and B cells react to a pathogen they've never seen, never knew existed, and were never inoculated against, and that you, or your doctors, in all their wisdom, could never have foreseen?
This is the infinity problem.
For years, this was the greatest mystery in immunology.
As I reported An Elegant Defense -- my book about the science of the immune system told through the lives of scientists and medical patients -- I was repeatedly struck by the profundity of this question. It is hard to overstate: how can we survive in a world with such myriad possible threats?

Matt Richtel's new book about the science of the immune system, An Elegant Defense, was published this month.
To further underscore the quandary, the immune system has to neutralize threats without killing the rest of the body. If the immune system could just kill the rest of the body too, the solution to the problem would be easy. Nuke the whole party. That obviously won't work if we are to survive. So the immune system has to be specific to the threat while also leaving most of our organism largely alone.
"God had two options," Dr. Mark Brunvand told me. "He could turn us into ten-foot-tall pimples, or he could give us the power to fight 10 to the 12th power different pathogens." That's a trillion potential bad actors. Why pimples? Pimples are filled with white blood cells, which are rich with immune system cells. In short, you could be a gigantic immune system and nothing else, or you could have some kind of secret power that allowed you to have all the other attributes of a human being—brain, heart, organs, limbs—and still somehow magically be able to fight infinite pathogens.
Dr. Brunvand is a retired Denver oncologist, one of the many medical authorities in the book – from wizened T-cell innovator Dr. Jacques Miller, to the finder of fever, Dr. Charles Dinarello, to his eminence Dr. Anthony Fauci at the National Institutes of Health to newly minted Nobel-Prize winner Jim Allison.
In the case of Dr. Brunvand, the oncologist also is integral to one of the book's narratives, a remarkable story of a friend of mine named Jason. Four years ago, he suffered late, late stage cancer, with 15 pounds of lymphoma growing in his back, and his oncologist put him into hospice. Then Jason became one of the first people ever to take an immunotherapy drug for lymphoma and his tumors disappeared. Through Jason's story, and a handful of other fascinating tales, I showcase how the immune system works.
There are two options for creating such a powerful immune system: we could be pimples or have some other magical power.
Dr. Brunvand had posited to me that there were two options for creating such a powerful and multifaceted immune system: we could be pimples or have some other magical power. You're not a pimple. So what was the ultimate solution?
Over the years, there were a handful of well-intentioned, thoughtful theories, but they strained to account for the inexplicable ability of the body to respond to virtually anything. The theories were complex and suffered from that peculiar side effect of having terrible names—like "side-chain theory" and "template-instructive hypothesis."
This was the background when along came Susumu Tonegawa.
***
Tonegawa was born in 1939, in the Japanese port city of Nagoya, and was reared during the war. Lucky for him, his father was moved around in his job, and so Tonegawa grew up in smaller towns. Otherwise, he might've been in Nagoya on May 14,1944, when the United States sent nearly 550 B-29 bombers to take out key industrial sites there and destroyed huge swaths of the city.
Fifteen years later, in 1959, Tonegawa was a promising student when a professor in Kyoto told him that he should go to the United States because Japan lacked adequate graduate training in molecular biology. A clear, noteworthy phenomenon was taking shape: Immunology and its greatest discoveries were an international affair, discoveries made through cooperation among the world's best brains, national boundaries be damned.
Tonegawa wound up at the University of California at San Diego, at a lab in La Jolla, "the beautiful Southern California town near the Mexican border." There, in multicultural paradise, he received his PhD, studying in the lab of Masaki Hayashi and then moved to the lab of Renato Dulbecco. Dr. Dulbecco was born in Italy, got a medical degree, was recruited to serve in World War II, where he fought the French and then, when Italian fascism collapsed, joined the resistance and fought the Germans. (Eventually, he came to the United States and in 1975 won a Nobel Prize for using molecular biology to show how viruses can lead, in some cases, to tumor creation.)
In 1970, Tonegawa—now armed with a PhD—faced his own immigration conundrum. His visa was set to expire by the end of 1970, and he was forced to leave the country for two years before he could return. He found a job in Switzerland at the Basel Institute for Immunology.
***
Around this time, new technology had emerged that allowed scientists to isolate different segments of an organism's genetic material. The technology allowed segments to be "cut" and then compared to one another. A truism emerged: If a researcher took one organism's genome and cut precisely the same segment over and over again, the resulting fragment of genetic material would match each time.
When you jump in that lake in a foreign land, filled with alien bugs, your body, astonishingly, well might have a defender that recognizes the creature.
This might sound obvious, but it was key to defining the consistency of an organism's genetic structure.
Then Tonegawa found the anomaly.
He was cutting segments of genetic material from within B cells. He began by comparing the segments from immature B cells, meaning, immune system cells that were still developing. When he compared identical segments in these cells, they yielded, predictably, identical fragments of genetic material. That was consistent with all previous knowledge.
But when he compared the segments to identical regions in mature B cells, the result was entirely different. This was new, distinct from any other cell or organism that had been studied. The underlying genetic material had changed.
"It was a big revelation," said Ruslan Medzhitov, a Yale scholar. "What he found, and is currently known, is that the antibody-encoding genes are unlike all other normal genes."
The antibody-encoding genes are unlike all other normal genes.
Yes, I used italics. Your immune system's incredible capabilities begin from a remarkable twist of genetics. When your immune system takes shape, it scrambles itself into millions of different combinations, random mixtures and blends. It is a kind of genetic Big Bang that creates inside your body all kinds of defenders aimed at recognizing all kinds of alien life forms.
So when you jump in that lake in a foreign land, filled with alien bugs, your body, astonishingly, well might have a defender that recognizes the creature.
Light the fireworks and send down the streamers!
As Tonegawa explored further, he discovered a pattern that described the differences between immature B cells and mature ones. Each of them shared key genetic material with one major variance: In the immature B cell, that crucial genetic material was mixed in with, and separated by, a whole array of other genetic material.
As the B cell matured into a fully functioning immune system cell, much of the genetic material dropped out. And not just that: In each maturing B cell, different material dropped out. What had begun as a vast array of genetic coding sharpened into this particular, even unique, strand of genetic material.
***
This is complex stuff. But a pep talk: This section is as deep and important as any in describing the wonder of the human body. Dear reader, please soldier on!
***
Researchers, who, eventually, sought a handy way to define the nature of the genetic change to the material of genes, labeled the key genetic material in an antibody with three initials: V, D, and J.
The letter V stands for variable. The variable part of the genetic material is drawn from hundreds of genes.
D stands for diversity, which is drawn from a pool of dozens of different genes.
And J is drawn from another half dozen genes.
In an immature B cell, the strands of V, D, and J material are in separate groupings, and they are separated by a relatively massive distance. But as the cell matures, a single, random copy of V remains, along with a single each of D and J, and all the other intervening material drops out. As I began to grasp this, it helped me to picture a line of genetic material stretching many miles. Suddenly, three random pieces step forward, and the rest drops away.
The combination of these genetic slices, grouped and condensed into a single cell, creates, by the power of math, trillions of different and virtually unique genetic codes.
In anticipation of threats from the unfathomable, our defenses evolved as infinity machines.
Or if you prefer a different metaphor, the body has randomly made hundreds of millions of different keys, or antibodies. Each fits a lock that is located on a pathogen. Many of these antibodies are combined such that they are alien genetic material—at least to us—and their locks will never surface in the human body. Some may not exist in the entire universe. Our bodies have come stocked with keys to the rarest and even unimaginable locks, forms of evil the world has not yet seen, but someday might. In anticipation of threats from the unfathomable, our defenses evolved as infinity machines.
"The discoveries of Tonegawa explain the genetic background allowing the enormous richness of variation among antibodies," the Nobel Prize committee wrote in its award to him years later, in 1987. "Beyond deeper knowledge of the basic structure of the immune system these discoveries will have importance in improving immunological therapy of different kinds, such as, for instance, the enforcement of vaccinations and inhibition of reactions during transplantation. Another area of importance is those diseases where the immune defense of the individual now attacks the body's own tissues, the so-called autoimmune diseases."
Indeed, these revelations are part of a period of time it would be fair to call the era of immunology, stretching from the middle of the 20th century to the present. During that period, we've come from sheer ignorance of the most basic aspects of the immune system to now being able to tinker under the hood with monoclonal antibodies and other therapies. And we are, in many ways, just at the beginning.