These technologies may help more animals and plants survive climate change

As the climate changes, the ripples will reach everywhere. Better data is needed for both plants and animals, and scientists are looking for genes that could allow crops to survive.
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are making us more vulnerable to infectious diseases by land and by sea - and how scientists are working on solutions.
Along the west coast of South Florida and the Keys, Florida Bay is a nursery for young Caribbean spiny lobsters, a favorite local delicacy. Growing up in small shallow basins, they are especially vulnerable to warmer, more saline water. Climate change has brought tidal floods, bleached coral reefs and toxic algal blooms to the state, and since the 1990s, the population of the Caribbean spiny lobster has dropped some 20 percent, diminishing an important food for snapper, grouper, and herons, as well as people. In 1999, marine ecologist Donald Behringer discovered the first known virus among lobsters, Panulirus argus virus—about a quarter of juveniles die from it before they mature.
“When the water is warm PaV1 progresses much more quickly,” says Behringer, who is based at the Emerging Pathogens Institute at the University of Florida in Gainesville.
Caribbean spiny lobsters are only one example of many species that are struggling in the era of climate change, both at sea and on land. As the oceans heat up, absorbing greenhouse gases and growing more acidic, marine diseases are emerging at an accelerated rate. Marine creatures are migrating to new places, and carrying pathogens with them. The latest grim report in the journal Science, states that if global warming continues at the current rate, the extinction of marine species will rival the Permian–Triassic extinction, sometimes called the “Great Dying,” when volcanoes poisoned the air and wiped out as much as 90 percent of all marine life 252 million years ago.
Similarly, on land, climate change has exposed wildlife, trees and crops to new or more virulent pathogens. Warming environments allow fungi, bacteria, viruses and infectious worms to proliferate in new species and locations or become more virulent. One paper modeling records of nearly 1,400 wildlife species projects that parasites will double by 2070 in the far north and in high-altitude places. Right now, we are seeing the effects most clearly on the fringes—along the coasts, up north and high in the mountains—but as the climate continues changing, the ripples will reach everywhere.
Few species are spared
On the Hawaiian Islands, mosquitoes are killing more songbirds. The dusky gray akikiki of Kauai and the chartreuse-yellow kiwikiu of Maui could vanish in two years, under assault from mosquitoes bearing avian malaria, according to a University of Hawaiʻi 2022 report. Previously, the birds could escape infection by roosting high in the cold mountains, where the pests couldn’t thrive, but climate change expanded the range of the mosquito and narrowed theirs.
Likewise, as more midge larvae survive over warm winters and breed better during drier summers, they bite more white-tailed deer, spreading often-fatal epizootic hemorrhagic disease. Especially in northern regions of the globe, climate change brings the threat of midges carrying blue tongue disease, a virus, to sheep and other animals. Tick-borne diseases like encephalitis and Lyme disease may become a greater threat to animals and perhaps humans.
"If you put all your eggs in one basket and then a pest comes a long, then you are more vulnerable to those risks," says Mehroad Ehsani, managing director of the food initiative in Africa for the Rockefeller Foundation. "Research is needed on resilient, climate smart, regenerative agriculture."
In the “thermal mismatch” theory of wildlife disease, cold-adapted species are at greater risk when their habitats warm, and warm-adapted species suffer when their habitats cool. Mammals can adjust their body temperature to adapt to some extent. Amphibians, fish and insects that cannot regulate body temperatures may be at greater risk. Many scientists see amphibians, especially, as canaries in the coalmine, signaling toxicity.
Early melting ice can foster disease. Climate models predict that the spring thaw will come ever-earlier in the lakes of the French Pyrenees, for instance, which traditionally stayed frozen for up to half the year. The tadpoles of the midwife toad live under the ice, where they are often infected with amphibian chytrid fungus. When a seven-year study tracked the virus in three species of amphibians in Pyrenees’s Lac Arlet, the research team found that, the earlier the spring thaw arrived, the more infection rates rose in common toads— , while remaining high among the midwife toads. But the team made another sad discovery: with early thaws, the common frog, which was thought to be free of the disease in Europe, also became infected with the fungus and died in large numbers.
Changing habitats affect animal behavior. Normally, spiny lobsters rely on chemical cues to avoid predators and sick lobsters. New conditions may be hampering their ability to “social distance”—which may help PaV1 spread, Behringer’s research suggests. Migration brings other risks. In April 2022, an international team led by scientists at Georgetown University announced the first comprehensive overview, published in the journal Nature, of how wild mammals under pressure from a changing climate may mingle with new populations and species—giving viruses a deadly opportunity to jump between hosts. Droughts, for example, will push animals to congregate at the few places where water remains.
Plants face threats also. At the timberline of the cold, windy, snowy mountains of the U.S. west, whitebark pine forests are facing a double threat, from white pine blister rust, a fungal disease, and multiplying pine beetles. “If we do nothing, we will lose the species,” says Robert Keane, a research ecologist for the U.S. Forest Service, based in Missoula, Montana. That would be a huge shift, he explains: “It’s a keystone species. There are over 110 animals that depend on it, many insects, and hundreds of plants.” In the past, beetle larvae would take two years to complete their lifecycle, and many died in frost. “With climate change, we're seeing more and more beetles survive, and sometimes the beetle can complete its lifecycle in one year,” he says.
Quintessential crops are under threat too
As some pathogens move north and new ones develop, they pose novel threats to the crops humans depend upon. This is already happening to wheat, coffee, bananas and maize.
Breeding against wheat stem rust, a fungus long linked to famine, was a key success in the mid-20th century Green Revolution, which brought higher yields around the world. In 2013, wheat stem rust reemerged in Germany after decades of absence. It ravaged both bread and durum wheat in Sicily in 2016 and has spread as far as England and Ireland. Wheat blast disease, caused by a different fungus, appeared in Bangladesh in 2016, and spread to India, the world’s second largest producer of wheat.
Insects, moths, worms, and coffee leaf rust—a fungus now found in all coffee-growing countries—threaten the livelihoods of millions of people who grow coffee, as well as everybody’s cup of joe. More heat, more intense rain, and higher humidity have allowed coffee leaf rust to cycle more rapidly. It has grown exponentially, overcoming the agricultural chemicals that once kept it under control.
To identify new diseases and fine-tune crops for resistance, scientists are increasingly relying on genomic tools.
Tar spot, a fungus native to Latin America that can cut corn production in half, has emerged in highland areas of Central Mexico and parts of the U.S.. Meanwhile, maize lethal necrosis disease has spread to multiple countries in Africa, notes Mehrdad Ehsani, Managing Director for the Food Initiative in Africa of the Rockefeller Foundation. The Cavendish banana, which most people eat today, was bred to be resistant to the fungus Panama 1. Now a new fungus, Panama 4, has emerged on every continent–including areas of Latin America that rely on the Cavendish for their income, reported a recent story in the Guardian. New threats are poised to emerge. Potato growers in the Andes Mountains have been shielded from disease because of colder weather at high altitude, but temperature fluxes and warming weather are expected to make this crop vulnerable to potato blight, found plant pathologist Erica Goss, at the Emerging Pathogens Institute.
Science seeks solutions
To protect food supplies in the era of climate change, scientists are calling for integrated global surveillance systems for crop disease outbreaks. “You can imagine that a new crop variety that is drought-tolerant could be susceptible to a pathogen that previous varieties had some resistance against,” Goss says. “Or a country suffers from a calamitous weather event, has to import seed from another country, and that seed is contaminated with a new pathogen or more virulent strain of an existing pathogen.” Researchers at the John Innes Center in Norwich and Aarhus University in Denmark have established ways to monitor wheat rust, for example.
Better data is essential, for both plants and animals. Historically, models of climate change predicted effects on plant pathogens based on mean temperatures, and scientists tracked plant responses to constant temperatures, explains Goss. “There is a need for more realistic tests of the effects of changing temperatures, particularly changes in daily high and low temperatures on pathogens,” she says.
To identify new diseases and fine-tune crops for resistance, scientists are increasingly relying on genomic tools. Goss suggests factoring the impact of climate change into those tools. Genomic efforts to select soft red winter wheat that is resistant to Fusarium head blight (FHB), a fungus that plagues farmers in the Southeastern U.S., have had early success. But temperature changes introduce a new factor.
A fundamental solution would be to bring back diversification in farming, says Ehsani. Thousands of plant species are edible, yet we rely on a handful. Wild relatives of domesticated crops are a store of possibly useful genes that may confer resistance to disease. The same is true for livestock. “If you put all your eggs in one basket and then a pest comes along, then you are more vulnerable to those risks. Research is needed on resilient, climate smart, regenerative agriculture,” Ehsani says.
Jonathan Sleeman, director of the U.S. Geological Survey National Wildlife Health Center, has called for data on wildlife health to be systematically collected and integrated with climate and other variables because more comprehensive data will result in better preventive action. “We have focused on detecting diseases,” he says, but a more holistic strategy would apply human public health concepts to assuring animal wellbeing. (For example, one study asked experts to draw a diagram of relationships of all the factors affecting the health of a particular group of caribou.) We must not take the health of plants and animals for granted, because their vulnerability inevitably affects us too, Sleeman says. “We need to improve the resilience of wildlife populations so they can withstand the impact of climate change.”
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
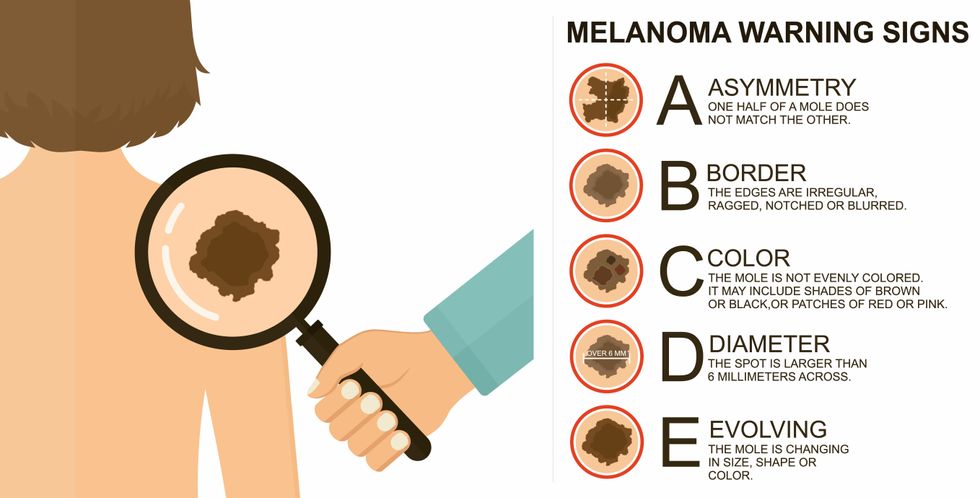
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

