Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
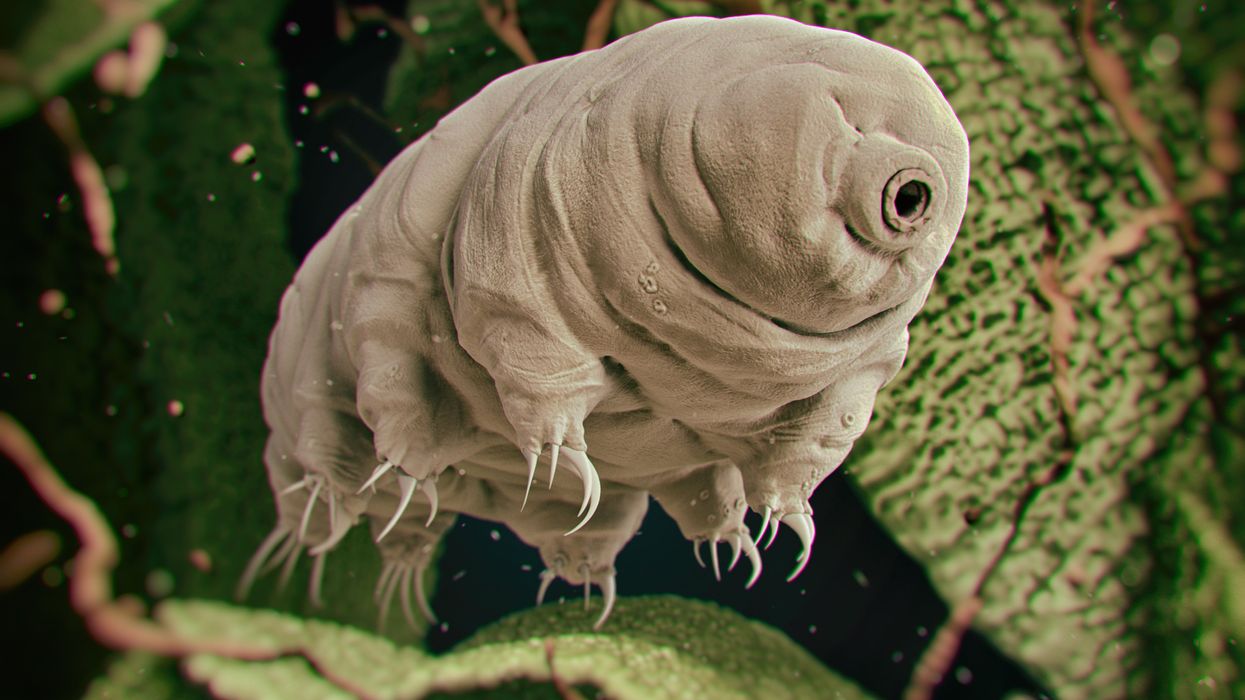
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
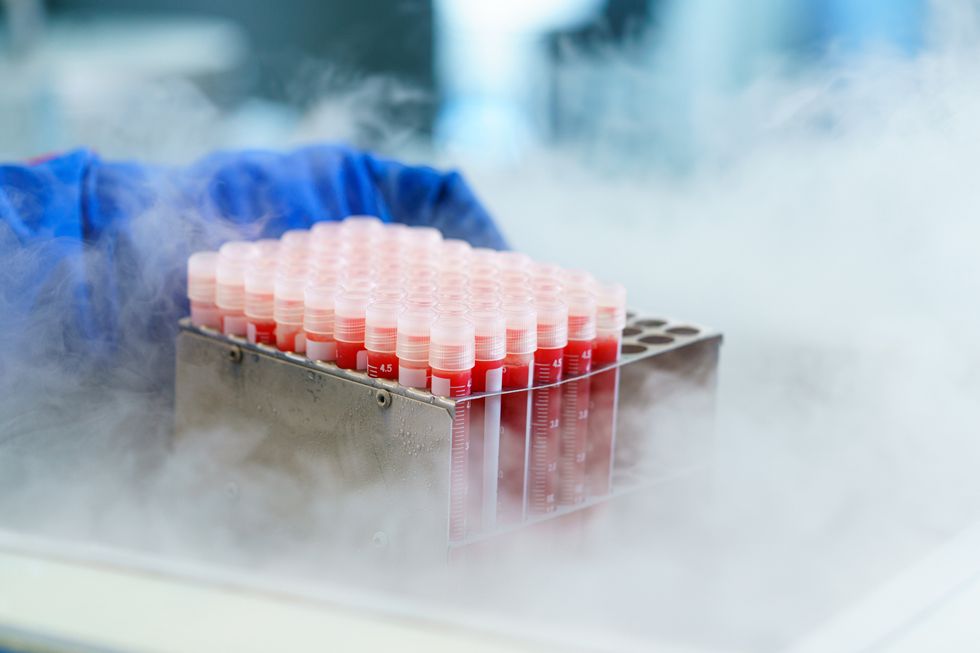
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
Fungus is the ‘New Black’ in Eco-Friendly Fashion
On the left, a Hermès bag made using fine mycelium as a leather alternative, made in partnership with the biotech company MycoWorks; on right, a sheet of mycelium "leather."
A natural material that looks and feels like real leather is taking the fashion world by storm. Scientists view mycelium—the vegetative part of a mushroom-producing fungus—as a planet-friendly alternative to animal hides and plastics.
Products crafted from this vegan leather are emerging, with others poised to hit the market soon. Among them are the Hermès Victoria bag, Lululemon's yoga accessories, Adidas' Stan Smith Mylo sneaker, and a Stella McCartney apparel collection.

The Adidas' Stan Smith Mylo concept sneaker, made in partnership with Bolt Threads, uses an alternative leather grown from mycelium; a commercial version is expected in the near future.
Adidas
Hermès has held presales on the new bag, says Philip Ross, co-founder and chief technology officer of MycoWorks, a San Francisco Bay area firm whose materials constituted the design. By year-end, Ross expects several more clients to debut mycelium-based merchandise. With "comparable qualities to luxury leather," mycelium can be molded to engineer "all the different verticals within fashion," he says, particularly footwear and accessories.
More than a half-dozen trailblazers are fine-tuning mycelium to create next-generation leather materials, according to the Material Innovation Initiative, a nonprofit advocating for animal-free materials in the fashion, automotive, and home-goods industries. These high-performance products can supersede items derived from leather, silk, down, fur, wool, and exotic skins, says A. Sydney Gladman, the institute's chief scientific officer.
That's only the beginning of mycelium's untapped prowess. "We expect to see an uptick in commercial leather alternative applications for mycelium-based materials as companies refine their R&D [research and development] and scale up," Gladman says, adding that "technological innovation and untapped natural materials have the potential to transform the materials industry and solve the enormous environmental challenges it faces."
In fewer than 10 days in indoor agricultural farms, "we grow large slabs of mycelium that are many feet wide and long. We are not confined to the shape or geometry of an animal."
Reducing our carbon footprint becomes possible because mycelium can flourish in indoor farms, using agricultural waste as feedstock and emitting inherently low greenhouse gas emissions. Carbon dioxide is the primary greenhouse gas. "We often think that when plant tissues like wood rot, that they go from something to nothing," says Jonathan Schilling, professor of plant and microbial biology at the University of Minnesota and a member of MycoWorks' Scientific Advisory Board.
But that assumption doesn't hold true for all carbon in plant tissues. When the fungi dominating the decomposition of plants fulfill their function, they transform a large portion of carbon into fungal biomass, Schilling says. That, in turn, ends up in the soil, with mycelium forming a network underneath that traps the carbon.
Unlike the large amounts of fossil fuels needed to produce styrofoam, leather and plastic, less fuel-intensive processing is involved in creating similar materials with a fungal organism. While some fungi consist of a single cell, others are multicellular and develop as very fine threadlike structures. A mass of them collectively forms a "mycelium" that can be either loose and low density or tightly packed and high density. "When these fungi grow at extremely high density," Schilling explains, "they can take on the feel of a solid material such as styrofoam, leather or even plastic."
Tunable and supple in the cultivation process, mycelium is also reliably sturdy in composition. "We believe that mycelium has some unique attributes that differentiate it from plastic-based and animal-derived products," says Gavin McIntyre, who co-founded Ecovative Design, an upstate New York-based biomaterials company, in 2007 with the goal of displacing some environmentally burdensome materials and making "a meaningful impact on our planet."
After inventing a type of mushroom-based packaging for all sorts of goods, in 2013 the firm ventured into manufacturing mycelium that can be adapted for textiles, he says, because mushrooms are "nature's recycling system."
The company aims for its material—which is "so tough and tenacious" that it doesn't require any plastic add-on as reinforcement—to be generally accessible from a pricing standpoint and not confined to a luxury space. The cost, McIntyre says, would approach that of bovine leather, not the more upscale varieties of lamb and goat skins.
Already, production has taken off by leaps and bounds. In fewer than 10 days in indoor agricultural farms, "we grow large slabs of mycelium that are many feet wide and long," he says. "We are not confined to the shape or geometry of an animal," so there's a much lower scrap rate.
Decreasing the scrap rate is a major selling point. "Our customers can order the pieces to the way that they want them, and there is almost no waste in the processing," explains Ross of MycoWorks. "We can make ours thinner or thicker," depending on a client's specific needs. Growing materials locally also results in a reduction in transportation, shipping, and other supply chain costs, he says.
Yet another advantage to making things out of mycelium is its biodegradability at the end of an item's lifecycle. When a pair of old sneakers lands in a compost pile or landfill, it decomposes thanks to microbial processes that, once again, involve fungi. "It is cool to think that the same organism used to create a product can also be what recycles it, perhaps building something else useful in the same act," says biologist Schilling. That amounts to "more than a nice business model—it is a window into how sustainability works in nature."
A product can be called "sustainable" if it's biodegradable, leaves a minimal carbon footprint during production, and is also profitable, says Preeti Arya, an assistant professor at the Fashion Institute of Technology in New York City and faculty adviser to a student club of the American Association of Textile Chemists and Colorists.
On the opposite end of the spectrum, products composed of petroleum-based polymers don't biodegrade—they break down into smaller pieces or even particles. These remnants pollute landfills, oceans, and rivers, contaminating edible fish and eventually contributing to the growth of benign and cancerous tumors in humans, Arya says.
Commending the steps a few designers have taken toward bringing more environmentally conscious merchandise to consumers, she says, "I'm glad that they took the initiative because others also will try to be part of this competition toward sustainability." And consumers will take notice. "The more people become aware, the more these brands will start acting on it."
A further shift toward mycelium-based products has the capability to reap tremendous environmental dividends, says Drew Endy, associate chair of bioengineering at Stanford University and president of the BioBricks Foundation, which focuses on biotechnology in the public interest.
The continued development of "leather surrogates on a scaled and sustainable basis will provide the greatest benefit to the greatest number of people, in perpetuity," Endy says. "Transitioning the production of leather goods from a process that involves the industrial-scale slaughter of vertebrate mammals to a process that instead uses renewable fungal-based manufacturing will be more just."
Can Biotechnology Take the Allergies Out of Cats?
From a special food to a vaccine and gene editing, new technologies may offer solutions for cat lovers with allergies.
Amy Bitterman, who teaches at Rutgers Law School in Newark, gets enormous pleasure from her three mixed-breed rescue cats, Spike, Dee, and Lucy. To manage her chronically stuffy nose, three times a week she takes Allegra D, which combines the antihistamine fexofenadine with the decongestant pseudoephedrine. Amy's dog allergy is rougher--so severe that when her sister launched a business, Pet Care By Susan, from their home in Edison, New Jersey, they knew Susan would have to move elsewhere before she could board dogs. Amy has tried to visit their brother, who owns a Labrador Retriever, taking Allegra D beforehand. But she began sneezing, and then developed watery eyes and phlegm in her chest.
"It gets harder and harder to breathe," she says.
Animal lovers have long dreamed of "hypo-allergenic" cats and dogs. Although to date, there is no such thing, biotechnology is beginning to provide solutions for cat-lovers. Cats are a simpler challenge than dogs. Dog allergies involve as many as seven proteins. But up to 95 percent of people who have cat allergies--estimated at 10 to 30 percent of the population in North America and Europe--react to one protein, Fel d1. Interestingly, cats don't seem to need Fel d1. There are cats who don't produce much Fel d1 and have no known health problems.
The current technologies fight Fel d1 in ingenious ways. Nestle Purina reached the market first with a cat food, Pro Plan LiveClear, launched in the U.S. a year and a half ago. It contains Fel d1 antibodies from eggs that in effect neutralize the protein. HypoCat, a vaccine for cats, induces them to create neutralizing antibodies to their own Fel d1. It may be available in the United States by 2024, says Gary Jennings, chief executive officer of Saiba Animal Health, a University of Zurich spin-off. Another approach, using the gene-editing tool CRISPR to create a medication that would splice out Fel d1 genes in particular tissues, is the furthest from fruition.
"Our goal was to ensure that whatever we do has no negative impact on the cat."
Customer demand is high. "We already have a steady stream of allergic cat owners contacting us desperate to have access to the vaccine or participate in the testing program," Jennings said. "There is a major unmet medical need."
More than a third of Americans own a cat (while half own a dog), and pet ownership is rising. With more Americans living alone, pets may be just the right amount of company. But the number of Americans with asthma increases every year. Of that group, some 20 to 30 percent have pet allergies that could trigger a possibly deadly attack. It is not clear how many pets end up in shelters because their owners could no longer manage allergies. Instead, allergists commonly report that their patients won't give up a beloved companion.
No one can completely avoid Fel d1, which clings to clothing and lands everywhere cat-owners go, even in schools and new homes never occupied by cats. Myths among cat-lovers may lead them to underestimate their own level of risk. Short hair doesn't help: the length of cat hair doesn't affect the production of Fel d1. Bathing your cat will likely upset it and accomplish little. Washing cuts the amount on its skin and fur only for two days. In one study, researchers measured the Fel d1 in the ambient air in a small chamber occupied by a cat—and then washed the cat. Three hours later, with the cat in the chamber again, the measurable Fel d1 in the air was lower. But this benefit was gone after 24 hours.
For years, the best option has been shots for people that prompt protective antibodies. Bitterman received dog and cat allergy injections twice a week as a child. However, these treatments require up to 100 injections over three to five years, and, as in her case, the effect may be partial or wear off. Even if you do opt for shots, treating the cat also makes sense, since you could protect more than one allergic member of your household and any allergic visitors as well.
An Allergy-Neutralizing Diet
Cats produce much of their Fel d1 in their saliva, which then spreads it to their fur when they groom, observed Nestle Purina immunologist Ebenezer Satyaraj. He realized that this made saliva—and therefore a cat's mouth--an unusually effective site for change. Hens exposed to Fel d1 produce their own antibodies, which survive in their eggs. The team coated LiveClear food with a powder form of these eggs; once in a cat's mouth, the chicken antibody binds to the Fel d1 in the cat's saliva, neutralizing it.
The results are partial: In a study with 105 cats, the level of active Fel d1 in their fur had dropped on average by 47 percent after ten weeks eating LiveClear. Cats that produced more Fel d1 at baseline had a more robust response, with a drop of up to 71 percent. A safety study found no effects on cats after six months on the diet. "Our goal was to ensure that whatever we do has no negative impact on the cat," Satyaraj said. Might a dogfood that minimizes dog allergens be on the way? "There is some early work," he said.
A Vaccine
This is a year when vaccines changed the lives of billions. Saiba's vaccine, HypoCat, delivers recombinant Fel d1 and the coat from a plant virus (the Cucumber mosaic virus) without any vital genetic information. The viral coat serves as a carrier. A cat would need shots once or twice a year to produce antibodies that neutralize Fel d1.
HypoCat works much like any vaccine, with the twist that the enemy is the cat's own protein. Is that safe? Saiba's team has followed 70 cats treated with the vaccine over two years and they remain healthy. Again the active Fel d1 doesn't disappear but diminishes. The team asked 10 people with cat allergies to report on their symptoms when they pet their vaccinated cats. Eight of them could pet their cat for nearly a half hour before their symptoms began, compared with an average of 17 minutes before the vaccine.
Jennings hopes to develop a HypoDog shot with a similar approach. However, the goal would be to target four or five proteins in one vaccine, and that increases the risk of hurting the dog. In the meantime, allergic dog-lovers considering an expensive breeder dog might think again: Independent research does not support the idea that any breed of dog produces less dander in the home. In fact, one well-designed study found that Spanish water dogs, Airedales, poodles and Labradoodles--breeds touted as hypo-allergenic--had significantly more of the most common allergen on their coat than an ordinary Lab and the control group.
Gene Editing
One day you might be able to bring your cat to the vet once a year for an injection that would modify specific tissues so they wouldn't produce Fel d1.
Nicole Brackett, a postdoctoral scientist at Viriginia-based Indoor Biotechnologies, which specializes in manufacturing biologics for allergy and asthma, most recently has used CRISPR to identify Fel d1 genetic sequences in cells from 50 domestic cats and 24 exotic ones. She learned that the sequences vary substantially from one cat to the next. This discovery, she says, backs up the observations that Fel d1 doesn't have a vital purpose.
The next step will be a CRISPR knockout of the relevant genes in cells from feline salivary glands, a prime source of Fel d1. Although the company is considering using CRISPR to edit the genes in a cat embryo and possibly produce a Fel d1-free cat, designer cats won't be its ultimate product. Instead, the company aims to produce injections that could treat any cat.
Reducing pet allergens at home could have a compound benefit, Indoor Biotechnologies founder Martin Chapman, an immunologist, notes: "When you dampen down the response to one allergen, you could also dampen it down to multiple allergens." As allergies become more common around the world, that's especially good news.

