Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
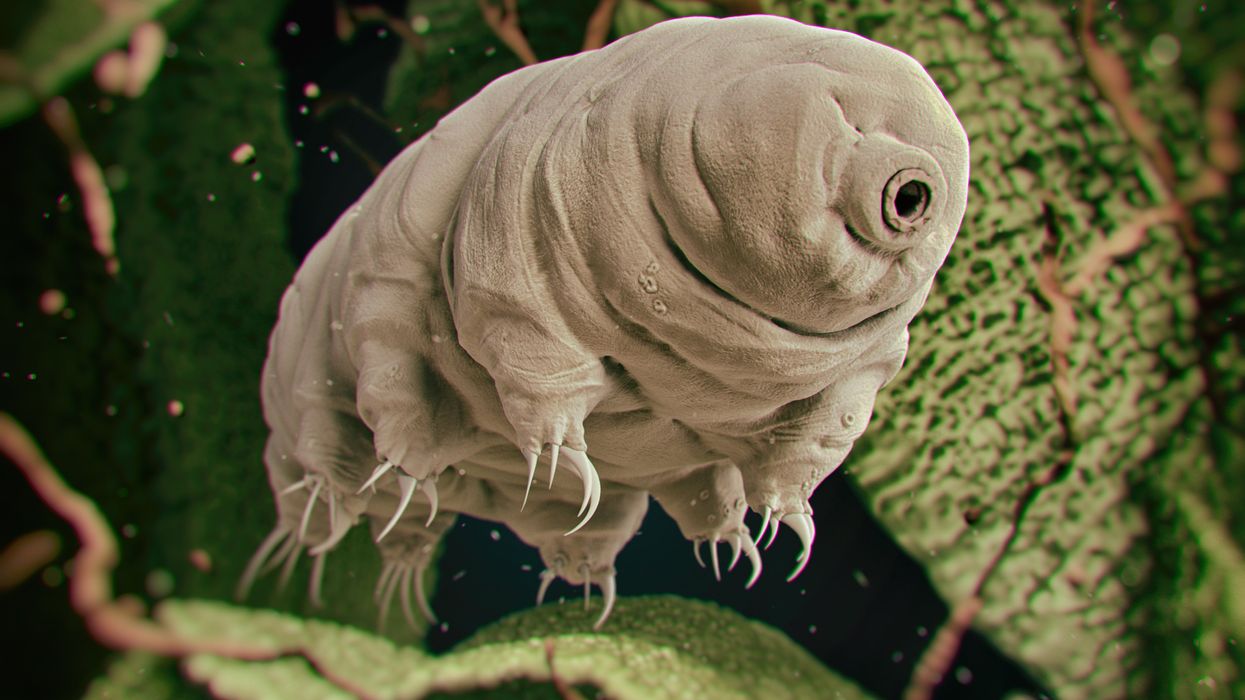
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
Henrietta Lacks' Cells Enabled Medical Breakthroughs. Is It Time to Finally Retire Them?
Henrietta Lacks, (1920-1951) unknowingly had her cells cultured and used in medical research.
For Victoria Tokarz, a third-year PhD student at the University of Toronto, experimenting with cells is just part of a day's work. Tokarz, 26, is studying to be a cell biologist and spends her time inside the lab manipulating muscle cells sourced from rodents to try to figure out how they respond to insulin. She hopes this research could someday lead to a breakthrough in our understanding of diabetes.
"People like to use HeLa cells because they're easy to use."
But in all her research, there is one cell culture that Tokarz refuses to touch. The culture is called HeLa, short for Henrietta Lacks, named after the 31-year-old tobacco farmer the cells were stolen from during a tumor biopsy she underwent in 1951.
"In my opinion, there's no question or experiment I can think of that validates stealing from and profiting off of a black woman's body," Tokarz says. "We're not talking about a reagent we created in a lab, a mixture of some chemicals. We're talking about a human being who suffered indescribably so we could profit off of her misfortune."
Lacks' suffering is something that, until recently, was not widely known. Born to a poor family in Roanoke, VA, Lacks was sent to live with her grandfather on the family tobacco farm at age four, shortly after the death of her mother. She gave birth to her first child at just fourteen, and two years later had another child with profound developmental disabilities. Lacks married her first cousin, David, in 1941 and the family moved to Maryland where they had three additional children.
But the real misfortune came in 1951, when Lacks told her cousins that she felt a hard "knot" in her womb. When Lacks went to Johns Hopkins hospital to have the knot examined, doctors discovered that the hard lump Henrietta felt was a rapidly-growing cervical tumor.
Before the doctors treated the tumor – inserting radium tubes into her vagina, in the hopes they could kill the cancer, Lacks' doctors clipped two tissue samples from her cervix, without Lacks' knowledge or consent. While it's considered widely unethical today, taking tissue samples from patients was commonplace at the time. The samples were sent to a cancer researcher at Johns Hopkins and Lacks continued treatment unsuccessfully until she died a few months later of metastatic cancer.
Lacks' story was not over, however: When her tissue sample arrived at the lab of George Otto Gey, the Johns Hopkins cancer researcher, he noticed that the cancerous cells grew at a shocking pace. Unlike other cell cultures that would die within a day or two of arriving at the lab, Lacks' cells kept multiplying. They doubled every 24 hours, and to this day, have never stopped.
Scientists would later find out that this growth was due to an infection of Human Papilloma Virus, or HPV, which is known for causing aggressive cancers. Lacks' cells became the world's first-ever "immortalized" human cell line, meaning that as long as certain environmental conditions are met, the cells can replicate indefinitely. Although scientists have cultivated other immortalized cell lines since then, HeLa cells remain a favorite among scientists due to their resilience, Tokarz says.
"People like to use HeLa cells because they're easy to use," Tokarz says. "They're easy to manipulate, because they're very hardy, and they allow for transection, which means expressing a protein in a cell that's not normally there. Other cells, like endothelial cells, don't handle those manipulations well."
Once the doctors at Johns Hopkins discovered that Lacks' cells could replicate indefinitely, they started shipping them to labs around the world to promote medical research. As they were the only immortalized cell line available at the time, researchers used them for thousands of experiments — some of which resulted in life-saving treatments. Jonas Salk's polio vaccine, for example, was manufactured using HeLa cells. HeLa cell research was also used to develop a vaccine for HPV, and for the development of in vitro fertilization and gene mapping. Between 1951 and 2018, HeLa cells have been cited in over 110,000 publications, according to a review from the National Institutes of Health.
But while some scientists like Tokarz are thankful for the advances brought about by HeLa cells, they still believe it's well past time to stop using them in research.
"Am I thankful we have a polio vaccine? Absolutely. Do I resent the way we came to have that vaccine? Absolutely," Tokarz says. "We could have still arrived at those same advances by treating her as the human being she is, not just a specimen."
Ethical considerations aside, HeLa is no longer the world's only available cell line – nor, Tokarz argues, are her cells the most suitable for every type of research. "The closer you can get to the physiology of the thing you're studying, the better," she says. "Now we have the ability to use primary cells, which are isolated from a person and put right into the culture dish, and those don't have the same mutations as cells that have been growing for 20 years. We didn't have the expertise to do that initially, but now we do."
Raphael Valdivia, a professor of molecular genetics and microbiology at Duke University School of Medicine, agrees that HeLa cells are no longer optimal for most research. "A lot of scientists are moving away from HeLa cells because they're so unstable," he says. "They mutate, they rearrange chromosomes to become adaptive, and different batches of cells evolve separately from each other. The HeLa cells in my lab are very different than the ones down the hall, and that means sometimes we can't replicate our results. We have to go back to an earlier batch of cells in the freezer and re-test."
Still, the idea of retiring the cells completely doesn't make sense, Valdivia says: "To some extent, you're beholden to previous research. You need to be able to confirm findings that happen in earlier studies, and to do that you need to use the same cell line that other researchers have used."
"Ethics is not black and white, and sometimes there's no such thing as a straightforward ethical or unethical choice."
"The way in which the cells were taken – without patient consent – is completely inappropriate," says Yann Joly, associate professor at the Faculty of Medicine in Toronto and Research Director at the Centre of Genomics and Policy. "The question now becomes, what can we do about it now? What are our options?"
While scientists are not able to erase what was done to Henrietta Lacks, Joly argues that retiring her cells is also non-consensual, assuming – maybe incorrectly – what Henrietta would have wanted, without her input. Additionally, Joly points out that other immortalized human cell lines are fraught with what some people consider to be ethical concerns as well, such as the human embryonic kidney cell line, commonly referred to as HEK-293, that was derived from an aborted female fetus. "Just because you're using another kind of cell doesn't mean it's devoid of ethical issue," he says.
Seemingly, the one thing scientists can agree on is that Henrietta Lacks was mistreated by the medical community. But even so, retiring her cells from medical research is not an obvious solution. Scientists are now using HeLa cells to better understand how the novel coronavirus affects humans, and this knowledge will inform how researchers develop a COVID-19 vaccine.
"Ethics is not black and white, and sometimes there's no such thing as a straightforward ethical or unethical choice," Joly says. "If [ethics] were that easy, nobody would need to teach it."
An intergalactic explorer on a hypothetical mission to Mars.
Social isolation. Strange pathogens outside. Strategic resource planning. Our Earthbound pandemic-driven social distancing could be mistaken for adapting to another, foreign planet. After all, we're donning all our protective apparel to go on an airplane or to the grocery store, nevertheless to just open our front door. Perhaps this is training for the world galactic visionaries Elon Musk, Jeff Bezos, and Richard Branson see in our future.
"There are parallels to the individual psychological experience, but from an operational standpoint, it is too different."
Ready to go live on Mars or something? Not so fast, experts say. The experience of shelter in place isn't parallel to being a space settler, or even an astronaut.
"Certain aspects are similar, but still, honestly, there are too many differences to say it preps us," says Angelo Vermeulen, co-founder of the art-science collective SEADS (Space Ecologies Art and Design) Network. In 2013, he served as a NASA crew commander for a four-month Mars-on-Earth mission, isolated in a geometric biodome with five others. "There are parallels to the individual psychological experience, but from an operational standpoint, it is too different. You don't need a spacesuit, aren't threatened by a thin atmosphere or worried about being overpowered by radiation."
Outside threats aside, we have a bigger experience gap: Most of us didn't see this pandemic coming and weren't trained to survive the current new normal. NASA astronauts get at least two years of basic training. We received none. Intergalactic explorers understand gravity, air pressure, and other important criteria based on decades of space knowledge. Alternatively, new novel coronavirus data is coming in real time, changing the threats, precautions, and needs dramatically. Things feel a little different when you're winging it.
Lastly, with respect to Apollo 13, space travelers have a timeline for when their experience will be over. There are mishaps, challenges and adjustments, but every well-supported journeyperson leaves Earth with an agenda (and a team back home to help keep them on track).
The pandemic, on the other hand, has no definitive end. It is unclear when a reliable vaccine will be readily available. It is also not known how long we should shelter-in-place, as pulling the trigger too early could bring another wave of illness. We are missing definitive milestones, which, Vermeulen says, would make our isolation experience easier to navigate. "When you're on a mission, the end date is always on the horizon. You can celebrate the midpoint and check off major milestones, which helps."
Also, unlike a kid pretending to be in a rocket, most of us didn't dream of one day being socially isolated for an indeterminate amount of time. "If you're ambitious and working in the field, then it is your goal in life to experience [space and the related isolation]," he says. "With the pandemic, though, nobody chose to do this."
[Editor's Note: This article was originally published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]

