The Ethics of Navigating Teen Gender Transitions

Transgender teen Theo Zachariah began identifying as trans at age 13, and now at 16, takes testosterone.
At first, Miriam Zachariah's teenage nephew Theo, who was born female, came out as gay. But he "presented as very gender fluid," she says, which suggested that he hadn't made "a clear choice one way or another."
Families, physicians, and psychologists have pondered whether it's better, neutral, or worse to postpone gender transitions until adulthood.
Zachariah decided to ask her nephew, "Do you think you might be trans?" While he answered "no," the question "broke something open for him," she recalls.
A month later, at age 13, he began identifying as trans. And at 14 1/2, he started undergoing gender transition with an endocrine-blocking injection. More recently, at age 16, he added testosterone injections, and soon he won't need the endocrine blocker any longer.
"His voice is deepening, and his muscle mass is growing," says Zachariah, a principal of two elementary schools in Toronto who became her nephew's legal guardian while he was starting to transition.
There are many medical and bioethical aspects associated with the transition to one's self-identified gender, especially when the process involves children and adolescents. Families, physicians, and psychologists have pondered whether it's better, neutral, or worse to postpone the transition until adulthood, while remaining cognizant of the potential consequences to puberty suppression with cross-sex hormones and the irreversibility of transgender surgeries.
Studies have found a higher prevalence of mental health issues among transgender and gender nonconforming youth, particularly if they are unable to express themselves in the self-identified gender. Research also has shown that transgender adults in the process of transitioning initially experienced worse mental health problems than their adolescent counterparts.
The Endocrine Society, a professional medical organization that provides recommendations for clinical practice, stipulates in its guidelines that the diagnosis of gender identity be limited to qualified mental health professionals for those under age 18. This is important because children are still evolving in their thought processes and capacity to articulate themselves, says endocrinologist Joshua Safer, inaugural executive director of the Center for Transgender Medicine and Surgery at the Icahn School of Medicine at Mount Sinai in New York.
A transition can begin safely in gradations, by allowing young children to experiment with haircuts and clothes of either gender before puberty. "If it just ends up being a stage of life, we haven't done anything permanent," says Safer, who is president of the United States Professional Association for Transgender Health as well as steering committee co-chair of TransNet, the international transgender research consortium.
After changes in appearance, the next step would be to try puberty blockers. Also used to halt precocious puberty, the injections are "a reasonably established intervention" for transgender youth, although there are some concerns that the drugs could interfere with bone health in the future, he says.
From a mental health standpoint, "hormones for youth who qualify for them have offered a tremendous boost in well-being and also a reduction in anxiety, depression, and suicidality that often plague transgender youth when they experience their bodies as totally discordant with their self-knowledge of their authentic gender," says psychologist Diane Ehrensaft, director of mental health in the Child and Adolescent Gender Center at Benioff Children's Hospital of the University of California at San Francisco.
Many of these youth have either known about or have been living in their authentic gender since early childhood; others discovered their true identities in adolescence, often with the onset of puberty, says Ehrensaft, associate professor of pediatrics. The effects of gender-affirming hormone treatments are at least partially reversible, she adds, whereas surgical procedures are irreversible. Regardless of reversibility, best practices include careful consideration of all interventions to ensure they are in a youth's best interests in promoting gender health and general well-being.
When a child exhibits signs of gender dysphoria, parents and guardians should at a minimum take these feelings seriously.
In determining readiness for a transgender operation, an assessment of maturity is as important as chronological age, says Loren Schechter, plastic surgeon and director of the Center for Gender Confirmation Surgery at Weiss Memorial Hospital in Chicago. With the consent of a parent or guardian, he commonly performs mastectomies on adolescents at age 17 and sometimes earlier, based on the clinical circumstances and along with a multidisciplinary team that includes a primary care provider and a mental health professional.
"Typically, before surgery, people have had the opportunity and time to consider their options," Schechter says, observing that "the incidence of regret or changing one's mind is extremely low." Others may opt to transition socially but not surgically. "We recognize that gender is not binary," he explains. Some individuals may not "discreetly fit into male or female" in how they perceive themselves.
When a child exhibits signs of gender dysphoria, parents and guardians should at a minimum take these feelings seriously, not dismiss them. They may want to enlist the assistance of a gender identity clinic to address the social environment and guide the child in exploring activities with the self-identified gender, says Kelly McBride Folkers, research associate in the Division of Medical Ethics at New York University School of Medicine.
At one end of the spectrum, some parents and guardians are overzealous in supporting their child's gender-identity pursuits while the youngster is still in an early phase of decision-making. On the flipside, other parents and guardians are not at all supportive, leaving the child at risk for long-term psychological effects, says Folkers, who is also associate director of the High School Bioethics Project at NYU, an educational program that aids teachers and students in examining ethical and conceptual concepts across various areas, one of which is gender.
"It's important to help children navigate through this process early, so that they have all of the social and familial support they need if and when they choose to seek medical options for gender affirmation later," she says.
There are various reasons why children and adolescents want to explore the opposite gender when they reach puberty. "It's a small percentage who will persist and insist and be consistent with that opposite gender identity," says Nicole Mihalopoulos, adolescent medicine physician and associate professor of pediatrics at the University of Utah School of Medicine in Salt Lake City.
Turning to a social work support system can help bring clarity for teens, parents, and guardians.
For those youth, it's appropriate to start the conversation about a medication to block puberty, but without actually promoting a hormonal transition to the opposite gender, in order for the child to further explore living as the opposite gender. "Children need to start at puberty because we need to know that their bodies are physiologically normal," Mihalopoulos says.
A lack of breast development in girls or a lack of testicular development in boys could point to an abnormality in the hypothalamus, pituitary gland, or ovaries/testicles. "That needs to be identified and corrected first," she explains, "before I would say, 'Let's start on the medical transition path of the alternate gender.' "
For parents and guardians, says Theo Zachariah's aunt Miriam, it's very tempting to misinterpret a child's struggling attempts to articulate being trans as an adolescent identity crisis. That's when turning to a social work support system can bring clarity. A youth mental health agency with experience in trans issues made a positive impact on Theo's family through one-on-one counseling and in groups for teens and parents.
"The dialogue they were able to engage in with my nephew, his mom and us," she says, was very instrumental "in helping us all figure out what to do and how to navigate the change."
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
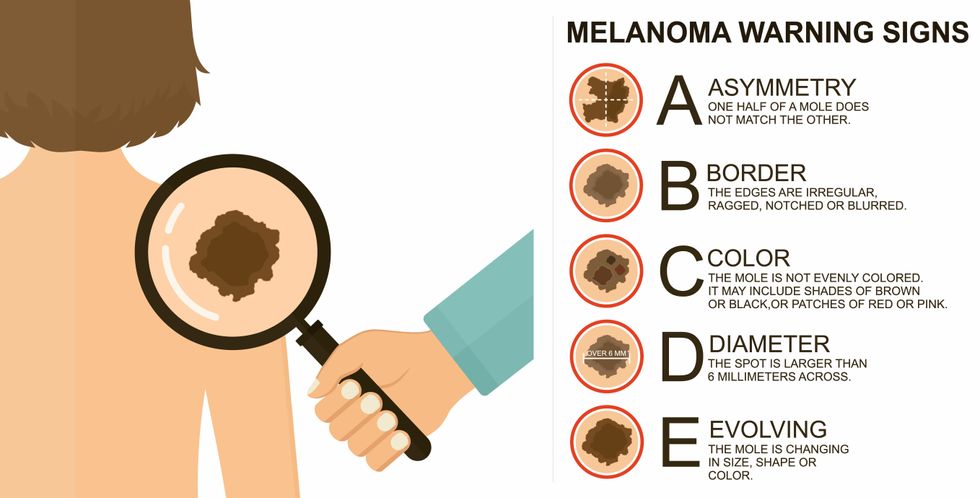
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

