The Ethics of Navigating Teen Gender Transitions

Transgender teen Theo Zachariah began identifying as trans at age 13, and now at 16, takes testosterone.
At first, Miriam Zachariah's teenage nephew Theo, who was born female, came out as gay. But he "presented as very gender fluid," she says, which suggested that he hadn't made "a clear choice one way or another."
Families, physicians, and psychologists have pondered whether it's better, neutral, or worse to postpone gender transitions until adulthood.
Zachariah decided to ask her nephew, "Do you think you might be trans?" While he answered "no," the question "broke something open for him," she recalls.
A month later, at age 13, he began identifying as trans. And at 14 1/2, he started undergoing gender transition with an endocrine-blocking injection. More recently, at age 16, he added testosterone injections, and soon he won't need the endocrine blocker any longer.
"His voice is deepening, and his muscle mass is growing," says Zachariah, a principal of two elementary schools in Toronto who became her nephew's legal guardian while he was starting to transition.
There are many medical and bioethical aspects associated with the transition to one's self-identified gender, especially when the process involves children and adolescents. Families, physicians, and psychologists have pondered whether it's better, neutral, or worse to postpone the transition until adulthood, while remaining cognizant of the potential consequences to puberty suppression with cross-sex hormones and the irreversibility of transgender surgeries.
Studies have found a higher prevalence of mental health issues among transgender and gender nonconforming youth, particularly if they are unable to express themselves in the self-identified gender. Research also has shown that transgender adults in the process of transitioning initially experienced worse mental health problems than their adolescent counterparts.
The Endocrine Society, a professional medical organization that provides recommendations for clinical practice, stipulates in its guidelines that the diagnosis of gender identity be limited to qualified mental health professionals for those under age 18. This is important because children are still evolving in their thought processes and capacity to articulate themselves, says endocrinologist Joshua Safer, inaugural executive director of the Center for Transgender Medicine and Surgery at the Icahn School of Medicine at Mount Sinai in New York.
A transition can begin safely in gradations, by allowing young children to experiment with haircuts and clothes of either gender before puberty. "If it just ends up being a stage of life, we haven't done anything permanent," says Safer, who is president of the United States Professional Association for Transgender Health as well as steering committee co-chair of TransNet, the international transgender research consortium.
After changes in appearance, the next step would be to try puberty blockers. Also used to halt precocious puberty, the injections are "a reasonably established intervention" for transgender youth, although there are some concerns that the drugs could interfere with bone health in the future, he says.
From a mental health standpoint, "hormones for youth who qualify for them have offered a tremendous boost in well-being and also a reduction in anxiety, depression, and suicidality that often plague transgender youth when they experience their bodies as totally discordant with their self-knowledge of their authentic gender," says psychologist Diane Ehrensaft, director of mental health in the Child and Adolescent Gender Center at Benioff Children's Hospital of the University of California at San Francisco.
Many of these youth have either known about or have been living in their authentic gender since early childhood; others discovered their true identities in adolescence, often with the onset of puberty, says Ehrensaft, associate professor of pediatrics. The effects of gender-affirming hormone treatments are at least partially reversible, she adds, whereas surgical procedures are irreversible. Regardless of reversibility, best practices include careful consideration of all interventions to ensure they are in a youth's best interests in promoting gender health and general well-being.
When a child exhibits signs of gender dysphoria, parents and guardians should at a minimum take these feelings seriously.
In determining readiness for a transgender operation, an assessment of maturity is as important as chronological age, says Loren Schechter, plastic surgeon and director of the Center for Gender Confirmation Surgery at Weiss Memorial Hospital in Chicago. With the consent of a parent or guardian, he commonly performs mastectomies on adolescents at age 17 and sometimes earlier, based on the clinical circumstances and along with a multidisciplinary team that includes a primary care provider and a mental health professional.
"Typically, before surgery, people have had the opportunity and time to consider their options," Schechter says, observing that "the incidence of regret or changing one's mind is extremely low." Others may opt to transition socially but not surgically. "We recognize that gender is not binary," he explains. Some individuals may not "discreetly fit into male or female" in how they perceive themselves.
When a child exhibits signs of gender dysphoria, parents and guardians should at a minimum take these feelings seriously, not dismiss them. They may want to enlist the assistance of a gender identity clinic to address the social environment and guide the child in exploring activities with the self-identified gender, says Kelly McBride Folkers, research associate in the Division of Medical Ethics at New York University School of Medicine.
At one end of the spectrum, some parents and guardians are overzealous in supporting their child's gender-identity pursuits while the youngster is still in an early phase of decision-making. On the flipside, other parents and guardians are not at all supportive, leaving the child at risk for long-term psychological effects, says Folkers, who is also associate director of the High School Bioethics Project at NYU, an educational program that aids teachers and students in examining ethical and conceptual concepts across various areas, one of which is gender.
"It's important to help children navigate through this process early, so that they have all of the social and familial support they need if and when they choose to seek medical options for gender affirmation later," she says.
There are various reasons why children and adolescents want to explore the opposite gender when they reach puberty. "It's a small percentage who will persist and insist and be consistent with that opposite gender identity," says Nicole Mihalopoulos, adolescent medicine physician and associate professor of pediatrics at the University of Utah School of Medicine in Salt Lake City.
Turning to a social work support system can help bring clarity for teens, parents, and guardians.
For those youth, it's appropriate to start the conversation about a medication to block puberty, but without actually promoting a hormonal transition to the opposite gender, in order for the child to further explore living as the opposite gender. "Children need to start at puberty because we need to know that their bodies are physiologically normal," Mihalopoulos says.
A lack of breast development in girls or a lack of testicular development in boys could point to an abnormality in the hypothalamus, pituitary gland, or ovaries/testicles. "That needs to be identified and corrected first," she explains, "before I would say, 'Let's start on the medical transition path of the alternate gender.' "
For parents and guardians, says Theo Zachariah's aunt Miriam, it's very tempting to misinterpret a child's struggling attempts to articulate being trans as an adolescent identity crisis. That's when turning to a social work support system can bring clarity. A youth mental health agency with experience in trans issues made a positive impact on Theo's family through one-on-one counseling and in groups for teens and parents.
"The dialogue they were able to engage in with my nephew, his mom and us," she says, was very instrumental "in helping us all figure out what to do and how to navigate the change."
Scientists are making machines, wearable and implantable, to act as kidneys
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon.
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen soon, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of the 2021 KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
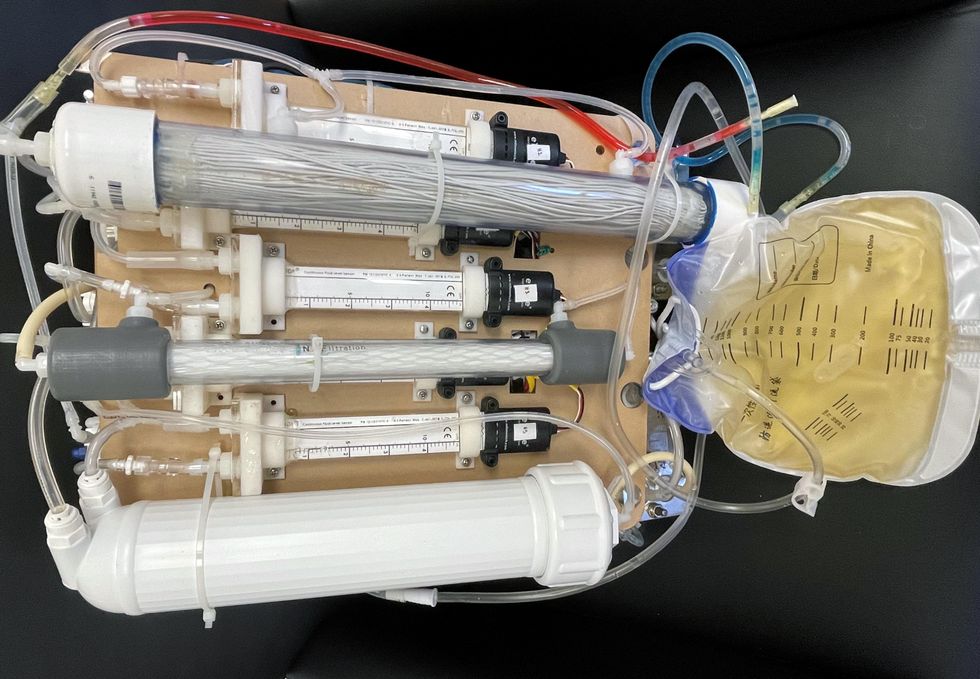
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
This article was first published by Leaps.org on May 5, 2022.
With this new technology, hospitals and pharmacies could make vaccines and medicines onsite
New research focuses on methods that could change medicine-making worldwide. The scientists propose bursting cells open, removing their DNA and using the cellular gears inside to make therapies.
Most modern biopharmaceutical medicines are produced by workhorse cells—typically bacterial but sometimes mammalian. The cells receive the synthesizing instructions on a snippet of a genetic code, which they incorporate into their DNA. The cellular machinery—ribosomes, RNAs, polymerases, and other compounds—read and use these instructions to build the medicinal molecules, which are harvested and administered to patients.
Although a staple of modern pharma, this process is complex and expensive. One must first insert the DNA instructions into the cells, which they may or may not uptake. One then must grow the cells, keeping them alive and well, so that they produce the required therapeutics, which then must be isolated and purified. To make this at scale requires massive bioreactors and big factories from where the drugs are distributed—and may take a while to arrive where they’re needed. “The pandemic showed us that this method is slow and cumbersome,” says Govind Rao, professor of biochemical engineering who directs the Center for Advanced Sensor Technology at the University of Maryland, Baltimore County (UMBC). “We need better methods that can work faster and can work locally where an outbreak is happening.”
Rao and his team of collaborators, which spans multiple research institutions, believe they have a better approach that may change medicine-making worldwide. They suggest forgoing the concept of using living cells as medicine-producers. Instead, they propose breaking the cells and using the remaining cellular gears for assembling the therapeutic compounds. Instead of inserting the DNA into living cells, the team burst them open, and removed their DNA altogether. Yet, the residual molecular machinery of ribosomes, polymerases and other cogwheels still functioned the way it would in a cell. “Now if you drop your DNA drug-making instructions into that soup, this machinery starts making what you need,” Rao explains. “And because you're no longer worrying about living cells, it becomes much simpler and more efficient.” The collaborators detail their cell-free protein synthesis or CFPS method in their recent paper published in preprint BioAxiv.
While CFPS does not use living cells, it still needs the basic building blocks to assemble proteins from—such as amino acids, nucleotides and certain types of enzymes. These are regularly added into this “soup” to keep the molecular factory chugging. “We just mix everything in as a batch and we let it integrate,” says James Robert Swartz, professor of chemical engineering and bioengineering at Stanford University and co-author of the paper. “And we make sure that we provide enough oxygen.” Rao likens the process to making milk from milk powder.
For a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology.
The idea of a cell-free protein synthesis is older than one might think. Swartz first experimented with it around 1997, when he was a chemical engineer at Genentech. While working on engineering bacteria to make pharmaceuticals, he discovered that there was a limit to what E. coli cells, the workhorse darling of pharma, could do. For example, it couldn’t grow and properly fold some complex proteins. “We tried many genetic engineering approaches, many fermentation, development, and environmental control approaches,” Swartz recalls—to no avail.
“The organism had its own agenda,” he quips. “And because everything was happening within the organism, we just couldn't really change those conditions very easily. Some of them we couldn’t change at all—we didn’t have control.”
It was out of frustration with the defiant bacteria that a new idea took hold. Could the cells be opened instead, so that the protein-forming reactions could be influenced more easily? “Obviously, we’d lose the ability for them to reproduce,” Swartz says. But that also meant that they no longer needed to keep the cells alive and could focus on making the specific reactions happen. “We could take the catalysts, the enzymes, and the more complex catalysts and activate them, make them work together, much as they would in a living cell, but the way we wanted.”
In 1998, Swartz joined Stanford, and began perfecting the biochemistry of the cell-free method, identifying the reactions he wanted to foster and stopping those he didn’t want. He managed to make the idea work, but for a variety of reasons—from the field’s general inertia to regulatory approval hurdles—the method hasn’t become mainstream. The pandemic rekindled interest in medicines that can be made quickly and easily, so it drew more attention to the technology. For their BioArxiv paper, the team tested the method by growing a specific antiviral protein called griffithsin.
First identified by Barry O’Keefe at National Cancer Institute over a decade ago, griffithsin is an antiviral known to interfere with many viruses’ ability to enter cells—including HIV, SARS, SARS-CoV-2, MERS and others. Originally isolated from the red algae Griffithsia, it works differently from antibodies and antibody cocktails.
Most antiviral medicines tend to target the specific receptors that viruses use to gain entry to the cells they infect. For example, SARS-CoV-2 uses the infamous spike protein to latch onto the ACE2 receptor of mammalian cells. The antibodies or other antiviral molecules stick to the spike protein, shutting off its ability to cling onto the ACE2 receptors. Unfortunately, the spike proteins mutate very often, so the medicines lose their potency. On the contrary, griffithsin has the ability to cling to the different parts of viral shells called capsids—namely to the molecules of mannose, a type of sugar. That extra stuff, glued all around the capsid like dead weight, makes it impossible for the virus to squeeze into the cell.
“Every time we have a vaccine or an antibody against a specific SARS-CoV-2 strain, that strain then mutates and so you lose efficacy,” Rao explains. “But griffithsin molecules glom onto the viral capsid, so the capsid essentially becomes a sticky mess and can’t enter the cell.” Mannose molecules also don’t mutate as easily as viruses’ receptors, so griffithsin-based antivirals do not have to be constantly updated. And because mannose molecules are found on many viruses’ capsids, it makes griffithsin “a universal neutralizer,” Rao explains.
“When griffithsin was discovered, we recognized that it held a lot of promise as a potential antiviral agent,” O’Keefe says. In 2010, he published a paper about griffithsin efficacy in neutralizing viruses of the corona family—after the first SARS outbreak in the early 2000s, the scientific community was interested in such antivirals. Yet, griffithsin is still not available as an off-the-shelf product. So during the Covid pandemic, the team experimented with synthesizing griffithsin using the cell-free production method. They were able to generate potent griffithsin in less than 24 hours without having to grow living cells.
The antiviral protein isn't the only type of medicine that can be made cell-free. The proteins needed for vaccine production could also be made the same way. “Such portable, on-demand drug manufacturing platforms can produce antiviral proteins within hours, making them ideal for combating future pandemics,” Rao says. “We would be able to stop the pandemic before it spreads.”
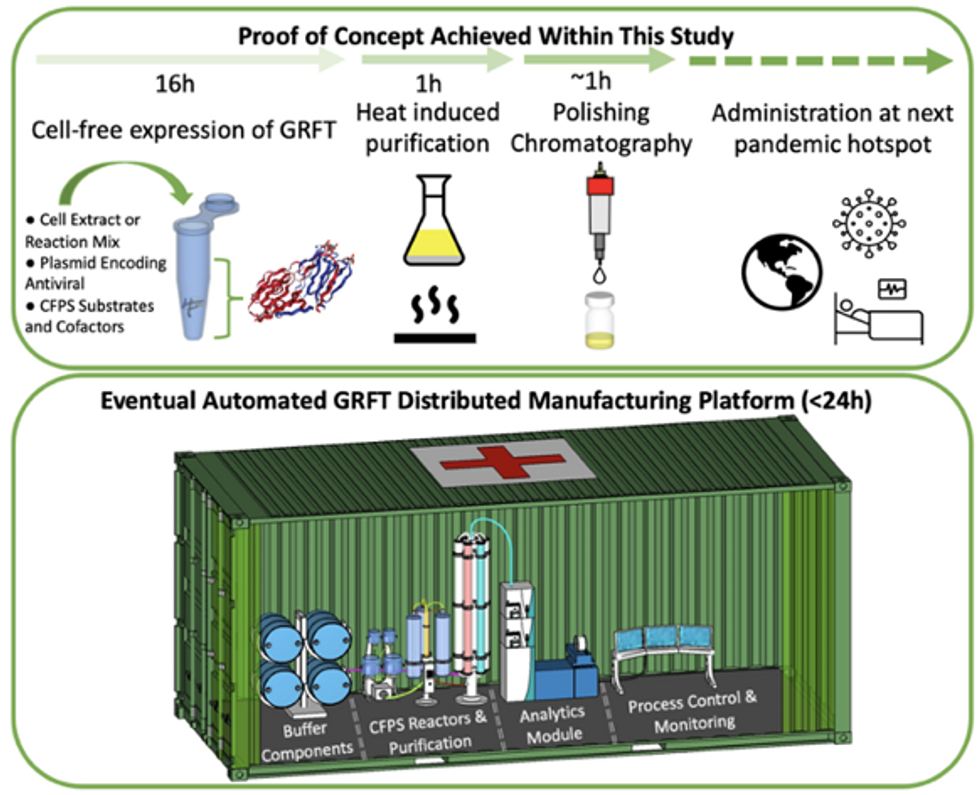
Top: Describes the process used in the study. Bottom: Describes how the new medicines and vaccines could be made at the site of a future viral outbreak.
Image courtesy of Rao and team, sourced from An approach to rapid distributed manufacturing of broad spectrumanti-viral griffithsin using cell-free systems to mitigate pandemics.
Rao’s idea is to perfect the technology to the point that any hospital or pharmacy can load up the media containing molecular factories, mix up the required amino acids, nucleotides and enzymes, and harvest the meds within hours. That will allow making medicines onsite and on demand. “That would be a self-contained production unit, so that you could just ship the production wherever the pandemic is breaking out,” says Swartz.
These units and the meds they produce, will, of course, have to undergo rigorous testing. “The biggest hurdles will be validating these against conventional technology,” Rao says. The biotech industry is risk-averse and prefers the familiar methods. But if this approach works, it may go beyond emergency situations and revolutionize the medicine-making paradigm even outside hospitals and pharmacies. Rao hopes that someday the method might become so mainstream that people may be able to buy and operate such reactors at home. “You can imagine a diabetic patient making insulin that way, or some other drugs,” Rao says. It would work not unlike making baby formula from the mere white powder. Just add water—and some oxygen, too.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.