The Promise of Pills That Know When You Swallow Them

A woman prepares to swallow a digital pill that can track whether she has taken her medication.
Dr. Sara Browne, an associate professor of clinical medicine at the University of California, San Diego, is a specialist in infectious diseases and, less formally, "a global health person." She often travels to southern Africa to meet with colleagues working on the twin epidemics of HIV and tuberculosis.
"This technology, in my opinion, is an absolute slam dunk for tuberculosis."
Lately she has asked them to name the most pressing things she can help with as a researcher based in a wealthier country. "Over and over and over again," she says, "the only thing they wanted to know is whether their patients are taking the drugs."
Tuberculosis is one of world's deadliest diseases; every year there are 10 million new infections and more than a million deaths. When a patient with tuberculosis is prescribed medicine to combat the disease, adherence to the regimen is important not just for the individual's health, but also for the health of the community. Poor adherence can lead to lengthier and more costly treatment and, perhaps more importantly, to drug-resistant strains of the disease -- an increasing global threat.
Browne is testing a new method to help healthcare workers track their patients' adherence with greater precision—close to exact precision even. They're called digital pills, and they involve a patient swallowing medicine as they normally would, only the capsule contains a sensor that—when it contacts stomach acid—transmits a signal to a small device worn on or near the body. That device in turn sends a signal to the patient's phone or tablet and into a cloud-based database. The fact that the pill has been swallowed has therefore been recorded almost in real time, and notice is available to whoever has access to the database.
"This technology, in my opinion, is an absolute slam dunk for tuberculosis," Browne says. TB is much more prevalent in poorer regions of the world—in Sub-Saharan Africa, for example—than in richer places like the U.S., where Browne's studies thus far have taken place. But when someone is diagnosed in the U.S., because of the risk to others if it spreads, they will likely have to deal with "directly observed therapy" to ensure that they take their medicines correctly.
DOT, as it's called, requires the patient to meet with a healthcare worker several days a week, or every day, so that the medicine intake can be observed in person -- an expensive and time-consuming process. Still, the Centers for Disease Control and Prevention website says (emphasis theirs), "DOT should be used for ALL patients with TB disease, including children and adolescents. There is no way to accurately predict whether a patient will adhere to treatment without this assistance."
Digital pills can help with both the cost and time involved, and potentially improve adherence in places where DOT is impossibly expensive. With the sensors, you can monitor a patient's adherence without a healthcare worker physically being in the room. Patients can live their normal lives and if they miss a pill, they can receive a reminder by text or a phone call from the clinic or hospital. "They can get on with their lives," said Browne. "They don't need the healthcare system to interrupt them."
A 56-year-old patient who participated in one of Browne's studies when he was undergoing TB treatment says that before he started taking the digital pills, he would go to the clinic at least once every day, except weekends. Once he switched to digital pills, he could go to work and spend time with his wife and children instead of fighting traffic every day to get to the clinic. He just had to wear a small patch on his abdomen, which would send the signal to a tablet provided by Browne's team. When he returned from work, he could see the results—that he'd taken the pill—in a database accessed via the tablet. (He could also see his heart rate and respiratory rate.) "I could do my daily activities without interference," he said.
Dr. Peter Chai, a medical toxicologist and emergency medicine physician at Brigham and Women's Hospital in Boston, is studying digital pills in a slightly different context, to help fight the country's opioid overdose crisis. Doctors like Chai prescribe pain medicine, he says, but then immediately put the onus on the patient to decide when to take it. This lack of guidance can lead to abuse and addiction. Patients are often told to take the meds "as needed." Chai and his colleagues wondered, "What does that mean to patients? And are people taking more than they actually need? Because pain is such a subjective experience."
The patients "liked the fact that somebody was watching them."
They wanted to see what "take as needed" actually led to, so they designed a study with patients who had broken a bone and come to the hospital's emergency department to get it fixed. Those who were prescribed oxycodone—a pharmaceutical opioid for pain relief—got enough digital pills to last one week. They were supposed to take the pills as needed, or as many as three pills per day. When the pills were ingested, the sensor sent a signal to a card worn on a lanyard around the neck.
Chai and his colleagues were able to see exactly when the patients took the pills and how many, and to detect patterns of ingestion more precisely than ever before. They talked to the patients after the seven days were up, and Chai said most were happy to be taking digital pills. The patients saw it as a layer of protection from afar. "They liked the fact that somebody was watching them," Chai said.
Both doctors, Browne and Chai, are in early stages of studies with patients taking pre-exposure prophylaxis, medicines that can protect people with a high-risk of contracting HIV, such as injectable drug users. Without good adherence, patients leave themselves open to getting the virus. If a patient is supposed to take a pill at 2 p.m. but the digital pill sensor isn't triggered, the healthcare provider can have an automatic message sent as a reminder. Or a reminder to one of the patient's friends or loved ones.
"Like Swallowing Your Phone"?
Deven Desai, an associate professor of law and ethics at Georgia Tech, says that digital pills sound like a great idea for helping with patient adherence, a big issue that self-reporting doesn't fully solve. He likes the idea of a physician you trust having better information about whether you're taking your medication on time. "On the surface that's just cool," he says. "That's a good thing." But Desai, who formerly worked as academic research counsel at Google, said that some of the same questions that have come up in recent years with social media and the Internet in general also apply to digital pills.
"Think of it like your phone, but you swallowed it," he says. "At first it could be great, simple, very much about the user—in this case, the patient—and the data is going between you and your doctor and the medical people it ought to be going to. Wonderful. But over time, phones change. They become 'smarter.'" And when phones and other technologies become smarter, he says, the companies behind them tend to expand the type of data they collect, because they can. Desai says it will be crucial that prescribers be completely transparent about who is getting the patients' data and for what purpose.
"We're putting stuff in our body in good faith with our medical providers, and what if it turned out later that all of a sudden someone was data mining or putting in location trackers and we never knew about that?" Desai asks. "What science has to realize is if they don't start thinking about this, what could be a wonderful technology will get killed."
Leigh Turner, an associate professor at the University of Minnesota's Center for Bioethics, agrees with Desai that digital pills have great promise, and also that there are clear reasons to be concerned about their use. Turner compared the pills to credit cards and social media, in that the data from them can potentially be stolen or leaked. One question he would want answered before the pills were normalized: "What kind of protective measures are in place to make sure that personal information isn't spilling out and being acquired by others or used by others in unexpected and unwanted ways?"
If digital pills catch on, some experts worry that they may one day not be a voluntary technology.
Turner also wonders who will have access to the pills themselves. Only those who can afford both the medicine plus the smartphones that are currently required for their use? Or will people from all economic classes have access? If digital pills catch on, he also worries they may one day not be a voluntary technology.
"When it comes to digital pills, it's not something that's really being foisted on individuals. It's more something that people can be informed of and can choose to take or not to take," he says. "But down the road, I can imagine a scenario where we move away from purely voluntary agreements to it becoming more of an expectation."
He says it's easy to picture a scenario in which insurance companies demand that patient medicinal intake data be tracked and collected or else. Refuse to have your adherence tracked and you risk higher rates or even overall coverage. Maybe patients who don't take the digital pills suffer dire consequences financially or medically. "Maybe it becomes beneficial as much to health insurers and payers as it is to individual patients," Turner says.
In November 2017, the FDA approved the first-ever digital pill that includes a sensor, a drug called Abilify MyCite, made by Otsuka Pharmaceutical Company. The drug, which is yet to be released, is used to treat schizophrenia, bipolar disorder, and depression. With a built-in sensor developed by Proteus Digital Health, patients can give their doctors permission to see when exactly they are taking, or not taking, their meds. For patients with mental illness, the ability to help them stick to their prescribed regime can be life-saving.
But Turner wonders if Abilify is the best drug to be a forerunner for digital pills. Some people with schizophrenia might be suffering from paranoia, and perhaps giving them a pill developed by a large corporation that sends data from their body to be tracked by other people might not be the best idea. It could in fact exacerbate their sense of paranoia.
The Bottom Line: Protect the Data
We all have relatives who have pillboxes with separate compartments for each day of the week, or who carry pillboxes that beep when it's time to take the meds. But that's not always good enough for people with dementia, mental illness, drug addiction, or other life situations that make it difficult to remember to take their pills. Digital pills can play an important role in helping these people.
"The absolute principle here is that the data has to belong to the patient."
The one time the patient from Browne's study forgot to take his pills, he got a beeping reminder from his tablet that he'd missed a dose. "Taking a medication on a daily basis, sometimes we just forget, right?" he admits. "With our very accelerated lives nowadays, it helps us to remember that we have to take the medications. So patients are able to be on top of their own treatment."
Browne is convinced that digital pills can help people in developing countries with high rates of TB and HIV, though like Turner and Desai she cautions that patients' data must be protected. "I think it can be a tremendous technology for patient empowerment and I also think if properly used it can help the medical system to support patients that need it," she said. "But the absolute principle here is that the data has to belong to the patient."
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of last year’s KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
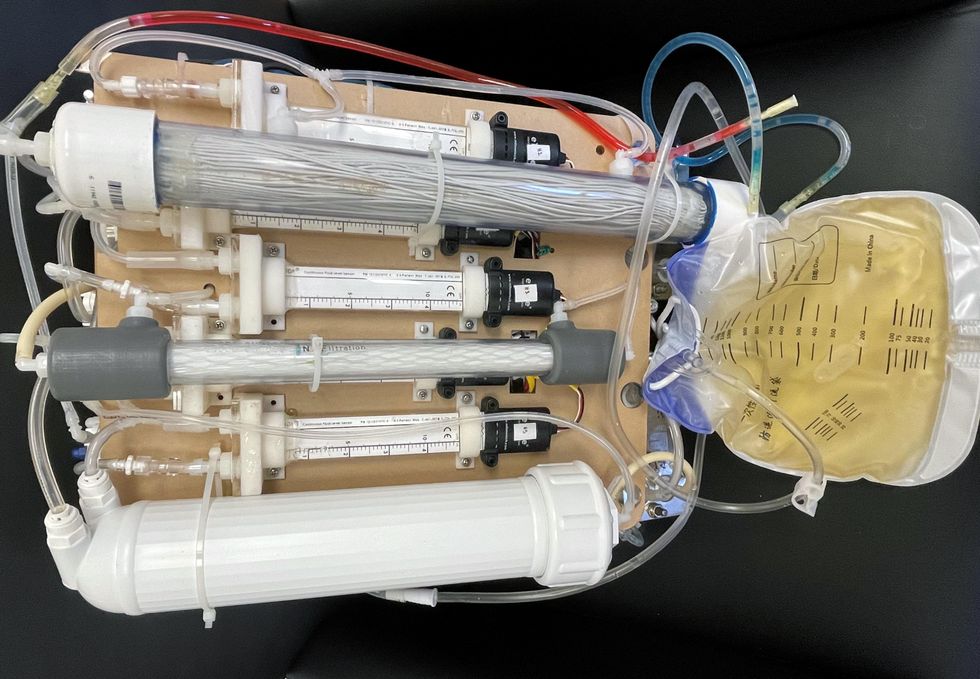
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
Today’s more than 20,000 mental health apps have a wide range of functionalities and business models. Many of them can be useful for depression.
Even before the pandemic created a need for more telehealth options, depression was a hot area of research for app developers. Given the high prevalence of depression and its connection to suicidality — especially among today’s teenagers and young adults who grew up with mobile devices, use them often, and experience these conditions with alarming frequency — apps for depression could be not only useful but lifesaving.
“For people who are not depressed, but have been depressed in the past, the apps can be helpful for maintaining positive thinking and behaviors,” said Andrea K. Wittenborn, PhD, director of the Couple and Family Therapy Doctoral Program and a professor in human development and family studies at Michigan State University. “For people who are mildly to severely depressed, apps can be a useful complement to working with a mental health professional.”
Health and fitness apps, in general, number in the hundreds of thousands. These are driving a market expected to reach $102.45 billion by next year. The mobile mental health app market is a small part of this but still sizable at $500 million, with revenues generated through user health insurance, employers, and direct payments from individuals.
Apps can provide data that health professionals cannot gather on their own. People’s constant interaction with smartphones and wearable devices yields data on many health conditions for millions of patients in their natural environments and while they go about their usual activities. Compared with the in-office measurements of weight and blood pressure and the brevity of doctor-patient interactions, the thousands of data points gathered unobtrusively over an extended time period provide a far better and more detailed picture of the person and their health.
At their most advanced level, apps for mental health, including depression, passively gather data on how the user touches and interacts with the mobile device through changes in digital biomarkers that relate to depressive symptoms and other conditions.
Building on three decades of research since early “apps” were used for delivering treatment manuals to health professionals, today’s more than 20,000 mental health apps have a wide range of functionalities and business models. Many of these apps can be useful for depression.
Some apps primarily provide a virtual connection to a group of mental health professionals employed or contracted by the app. Others have options for meditation, sleeping or, in the case of industry leaders Calm and Headspace, overall well-being. On the cutting edge are apps that detect changes in a person’s use of mobile devices and their interactions with them.
Apps such as AbleTo, Happify Health, and Woebot Health focus on cognitive behavioral therapy, a type of counseling with proven potential to change a person’s behaviors and feelings. “CBT has been demonstrated in innumerable studies over the last several decades to be effective in the treatment of behavioral health conditions such as depression and anxiety disorders,” said Dr. Reena Pande, chief medical officer at AbleTo. “CBT is intended to be delivered as a structured intervention incorporating key elements, including behavioral activation and adaptive thinking strategies.”
These CBT skills help break the negative self-talk (rumination) common in patients with depression. They are taught and reinforced by some self-guided apps, using either artificial intelligence or programmed interactions with users. Apps can address loneliness and isolation through connections with others, even when a symptomatic person doesn’t feel like leaving the house.
At their most advanced level, apps for mental health, including depression, passively gather data on how the user touches and interacts with the mobile device through changes in “digital biomarkers” that can be associated with onset or worsening of depressive symptoms and other cognitive conditions. In one study, Mindstrong Health gathered a year’s worth of data on how people use their smartphones, such as scrolling through articles, typing and clicking. Mindstrong, whose founders include former leaders of the National Institutes of Health, modeled the timing and order of these actions to make assessments that correlated closely with gold-standard tests of cognitive function.
National organizations of mental health professionals have been following the expanding number of available apps over the years with keen interest. App Advisor is an initiative of the American Psychiatric Association that helps psychiatrists and other mental health professionals navigate the issues raised by mobile health technology. App Advisor does not rate or recommend particular apps but rather provides guidance about why apps should be assessed and how health professionals can do this.
A website that does review mental health apps is One Mind Psyber Guide, an independent nonprofit that partners with several national organizations. One Mind users can select among numerous search terms for the condition and therapeutic approach of interest. Apps are rated on a five-point scale, with reviews written by professionals in the field.
Do mental health apps related to depression have the kind of safety and effectiveness data required for medications and other medical interventions? Not always — and not often. Yet the overall results have shown early promise, Wittenborn noted.
“Studies that have attempted to detect depression from smartphone and wearable sensors [during a single session] have ranged in accuracy from about 86 to 89 percent,” Wittenborn said. “Studies that tried to predict changes in depression over time have been less accurate, with accuracy ranging from 59 to 85 percent.”
The Food and Drug Administration encourages the development of apps and has approved a few of them—mostly ones used by health professionals—but it is generally “hands off,” according to the American Psychiatric Association. The FDA has published a list of examples of software (including programming of apps) that it does not plan to regulate because they pose low risk to the public. First on the list is software that helps patients with diagnosed psychiatric conditions, including depression, maintain their behavioral coping skills by providing a “Skill of the Day” technique or message.
On its App Advisor site, the American Psychiatric Association says mental health apps can be dangerous or cause harm in multiple ways, such as by providing false information, overstating the app’s therapeutic value, selling personal data without clearly notifying users, and collecting data that isn’t relevant to mental health.
Although there is currently reason for caution, patients may eventually come to expect mental health professionals to recommend apps, especially as their rating systems, features and capabilities expand. Through such apps, patients might experience more and higher quality interactions with their mental health professionals. “Apps will continue to be refined and become more effective through future research,” said Wittenborn. “They will become more integrated into practice over time.”