The Science Sleuth Holding Fraudulent Research Accountable
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

Outside whistleblower Elisabeth Bik scrutinizes newly published scientific papers for misleading images and data.
Introduction by Mary Inman, Whistleblower Attorney
For most people, when they see the word "whistleblower," the image that leaps to mind is a lone individual bravely stepping forward to shine a light on misconduct she has witnessed first-hand. Meryl Streep as Karen Silkwood exposing safety violations observed while working the line at the Kerr-McGee plutonium plant. Matt Damon as Mark Whitacre in The Informant!, capturing on his pocket recorder clandestine meetings between his employer and its competitors to fix the price of lysine. However, a new breed of whistleblower is emerging who isn't at the scene of the crime but instead figures it out after the fact through laborious review of publicly available information and expert analysis. Elisabeth Bik belongs to this new class of whistleblower.
"There's this delicate balance where on one hand we want to spread results really fast as scientists, but on the other hand, we know it's incomplete, it's rushed and it's not great."
Using her expertise as a microbiologist and her trained eye, Bik studies publicly available scientific papers to sniff out potential irregularities in the images that suggest research fraud, later seeking retraction of the offending paper from the journal's publisher. There's no smoking gun, no first-hand account of any kind. Just countless hours spent reviewing scores of scientific papers and Bik's skills and dedication as a science fraud sleuth.
While Bik's story may not as readily lend itself to the big screen, her work is nonetheless equally heroic. By tirelessly combing scientific papers to expose research fraud, Bik is playing a vital role in holding the scientific publishing process accountable and ensuring that misleading information does not spread unchecked. This is important work in any age, but particularly so in the time of COVID, where we can ill afford the setbacks and delays of scientists building on false science. In the present climate, where science is politicized and scientific principles are under attack, strong voices like Bik's must rise above the din to ensure the scientific information we receive, and our governments act upon, is accurate. Our health and wellbeing depend on it.
Whistleblower outsiders like Bik are challenging the traditional concept of what it means to be a whistleblower. Fortunately for us, the whistleblower community is a broad church. As with most ecosystems, we all benefit from a diversity of voices —whistleblower insiders and outsiders alike. What follows is an illuminating conversation between Bik, and Ivan Oransky, the co-founder of Retraction Watch, an influential blog that reports on retractions of scientific papers and related topics. (Conversation facilitated by LeapsMag Editor-in-Chief Kira Peikoff)

Elisabeth Bik and Ivan Oransky.
(Photo credits Michel & Co Photography, San Jose, CA and Elizabeth Solaka)
Ivan
I'd like to hear your thoughts, Elisabeth, on an L.A. Times story, which was picking up a preprint about mutations and the novel coronavirus, alleging that the virus is mutating to become more infectious – even though this conclusion wasn't actually warranted.
Elisabeth
A lot of the news around it is picking up on one particular side of the story that is maybe not that much exaggerated by the scientists. I don't think this paper really showed that the mutations were causing the virus to be more virulent. Some of these viruses continuously mutate and mutate and mutate, and that doesn't necessarily make a strain more virulent. I think in many cases, a lot of people want to read something in a paper that is not actually there.
Ivan
The tone level, everything that's being published now, it's problematic. It's being rushed, here it wasn't even peer-reviewed. But even when they are peer-reviewed, they're being peer-reviewed by people who often aren't really an expert in that particular area.
Elisabeth
That's right.
Ivan
To me, it's all problematic. At the same time, it's all really good that it's all getting out there. I think that five or 10 years ago, or if we weren't in a pandemic, maybe that paper wouldn't have appeared at all. It would have maybe been submitted to a top-ranked journal and not have been accepted, or maybe it would have been improved during peer review and bounced down the ladder a bit to a lower-level journal.
Yet, now, because it's about coronavirus, it's in a major newspaper and, in fact, it's getting critiqued immediately.
Maybe it's too Pollyanna-ish, but I actually think that quick uploading is a good thing. The fear people have about preprint servers is based on this idea that the peer-reviewed literature is perfect. Once it is in a peer-reviewed journal, they think it must have gone through this incredible process. You're laughing because-
Elisabeth
I am laughing.
Ivan
You know it's not true.
Elisabeth
Yes, we both know that. I agree and I think in this particular situation, a pandemic that is unlike something our generation has seen before, there is a great, great need for fast dissemination of science.
If you have new findings, it is great that there is a thing called a preprint server where scientists can quickly share their results, with, of course, the caveat that it's not peer-reviewed yet.
It's unlike the traditional way of publishing papers, which can take months or years. Preprint publishing is a very fast way of spreading your results in a good way so that is what the world needs right now.
On the other hand, of course, there's the caveat that these are brand new results and a good scientist usually thinks about their results to really interpret it well. You have to look at it from all sides and I think with the rushed publication of preprint papers, there is no such thing as carefully thinking about what results might mean.
So there's this delicate balance where on one hand we want to spread results really fast as scientists, but on the other hand, we know it's incomplete, it's rushed and it's not great. This might be hard for the general audience to understand.
Ivan
I still think the benefits of that dissemination are more positive than negative.
Elisabeth
Right. But there's also so many papers that come out now on preprint servers and most of them are not that great, but there are some really good studies in there. It's hard to find those nuggets of really great papers. There's just a lot of papers that come out now.
Ivan
Well, you've made more than a habit of finding problems in papers. These are mostly, of course, until now published papers that you examined, but what is this time like for you? How is it different?
Elisabeth
It's different because in the beginning I looked at several COVID-19-related papers that came out and wrote some critiques about it. I did experience a lot of backlash because of that. So I felt I had to take a break from social media and from writing about COVID-19.
I focused a little bit more on other work because I just felt that a lot of these papers on COVID-19 became so politically divisive that if you tried to be a scientist and think critically about a paper, you were actually assigned to a particular political party or to be against other political parties. It's hard for me to be sucked into the political discussion and to the way that our society now is so completely divided into two camps that seem to be not listening to each other.
Ivan
I was curious about that because I've followed your work for a number of years, as you know, and certainly you have had critics before. I'm thinking of the case in China that you uncovered, the leading figure in the Chinese Academy who was really a powerful political figure in addition to being a scientist.
Elisabeth
So that was a case in which I found a couple of papers at first from a particular group in China, and I was just posting on a website called PubPeer, where you can post comments, concerns about papers. And in this case, these were image duplication issues, which is my specialty.
I did not realize that the group I was looking at at that moment was led by one of the highest ranked scientists in China. If I had known that, I would probably not have posted that under my full name, but under a pseudonym. Since I had already posted, some people were starting to send me direct messages on Twitter like, "OMG, the guy you're posting about now is the top scientist in China so you're going to have a lot of backlash."
Then I decided I'll just continue doing this. I found a total of around 50 papers from this group and posted all of them on PubPeer. That story quickly became a very popular story in China: number two on Sina Weibo, a social media site in China.
I was surprised it wasn't suppressed by the Chinese government, it was actually allowed by journalists that were writing about it, and I didn't experience a lot of backlash because of that.
Actually the Chinese doctor wrote me an email saying that he appreciated my feedback and that he would look into these cases. He sent a very polite email so I sent him back that I appreciated that he would look into these cases and left it there.
Ivan
There are certain subjects that I know when we write about them in Retraction Watch, they have tended in the past to really draw a lot of ire. I'm thinking anything about vaccines and autism, anything about climate change, stem cell research.
For a while that last subject has sort of died down. But now it's become a highly politically charged atmosphere. Do you feel that this pandemic has raised the profile of people such as yourself who we refer to as scientific sleuths, people who look critically and analytically at new research?
Elisabeth
Yeah, some people. But I'm also worried that some people who are great scientists and have shown a lot of critical thinking are being attacked because of that. If you just look at what happened to Dr. Fauci, I think that's a prime example. Where somebody who actually is very knowledgeable and very cautious of new science has not been widely accepted as a great leader, in our country at least. It's sad to see that. I'm just worried how long he will be at his position, to be honest.
Ivan
We noticed a big uptick in our traffic in the last few days to Retraction Watch and it turns out it was because someone we wrote about a number of years ago has really hopped on the bandwagon to try and discredit and even try to have Dr. Fauci fired.
It's one of these reminders that the way people think about scientists has, in many cases, far more to do with their own history or their own perspective going in than with any reality or anything about the science. It's pretty disturbing, but it's not a new thing. This has been happening for a while.
You can go back and read sociologists of science from 50-60 years ago and see the same thing, but I just don't think that it's in the same way that it is now, maybe in part because of social media.
Elisabeth
I've been personally very critical about several studies, but this is the first time I've experienced being attacked by trolls and having some nasty websites written about me. It is very disturbing to read.
"I don't think that something that's been peer-reviewed is perfect and something that hasn't been peer reviewed, you should never bother reading it."
Ivan
It is. Yet you have been a fearless and vocal critic of some very high-profile papers, like the infamous French study about hydroxychloroquine.
Elisabeth
Right, the paper that came out was immediately tweeted by the President of the United States. At first I thought it was great that our President tweeted about science! I thought that was a major breakthrough. I took a look at this paper.
It had just come out that day, I believe. The first thing I noticed is that it was accepted within 24 hours of being submitted to the journal. It was actually published in a journal where one of the authors is the editor-in-chief, which is a huge conflict of interest, but it happens.
But in this particular case, there were also a lot of flaws with the study and that, I think, should have been caught during peer review. The paper was first published on a preprint server and then within 24 hours or so it was published in that paper, supposedly after peer review.
There were very few changes between the preprint version and the peer review paper. There were just a couple of extra lines, extra sentences added here and there, but it wasn't really, I think, critically looked at. Because there were a lot of things that I thought were flaws.
Just to go over a couple of them. This paper showed supposedly that people who were treated with hydroxychloroquine and azithromycin were doing much better by clearing their virus much faster than people who were not treated with these drugs.
But if you look carefully at the paper there were a couple of people who were left out of the study. So they were treated with hydroxychloroquine, but they were not shown in the end results of the paper. All six people who were treated with the drug combination were clearing the virus within six days, but there were a couple of others who were left out of the study. They also started the drug combination, but they stopped taking the drugs for several reasons and three of them were admitted to the intensive care, one died, one had some side effects and one apparently walked out of the hospital.
They were left out of the study but they were actually not doing very well with the drug combination. It's not very good science if you leave out people who don't do very well with your drug combination in your study. That was one of my biggest critiques of the paper.
Ivan
What struck us about that case was, in addition to what you, of course, mentioned, the fact that Trump tweeted it and was talking about hydroxychloroquine, was that it seemed to be a perfect example of, "well, it was in a peer review journal." Yeah, it was a preprint first, but, well, it's a peer review journal. And yet, as you point out, when you look at the history of the paper, it was accepted in 24 hours.
If you talk to most scientists, the actual act of a peer review, once you sit down to do it and can concentrate, a good one takes, again, these are averages, but four hours, a half a day is not unreasonable. So you had to find three people who could suddenly review this paper. As you pointed out, it was in a journal where one of the authors was editor.
Then some strange things also happened, right? The society that actually publishes the journal, they came out with a statement saying this wasn't up to our standards, which is odd. Then Elsevier came in, they're the ones who are actually contracted to publish the journal for the society. They said, basically, "Oh, we're going to look into this now too."
It just makes you wonder what happened before the paper was actually published. All the people who were supposed to have been involved in doing the peer review or checking on it are clearly very distraught about what actually happened. It's that scene from Casablanca, "I'm shocked, shocked there's gambling going on here." And then, "Your winnings, sir."
Elisabeth
Yes.
Ivan
And I don't actually blame the public, I don't blame reporters for getting a bit confused about what it all means and what they should trust. I don't think trust is a binary any more than anything else is a binary. I don't think that something that's been peer-reviewed is perfect and something that hasn't been peer reviewed, you should never bother reading it. I think everything is much more gray.
Yet we've turned things into a binary. Even if you go back before coronavirus, coffee is good for you, coffee is bad for you, red wine, chocolate, all the rest of it. A lot of that is because of this sort of binary construct of the world for journalists, frankly, for scientists that need to get their next grants. And certainly for the general public, they want answers.
On the one hand, if I had to choose what group of experts, or what field of human endeavor would I trust with finding the answer to a pandemic like this, or to any crisis, it would absolutely be scientists. Hands down. This is coming from someone who writes about scientific fraud.
But on the other hand, that means that if scientists aren't clear about what they don't know and about the nuances and about what the scientific method actually allows us to do and learn, that just sets them up for failure. It sets people like Dr. Fauci up for failure.
Elisabeth
Right.
Ivan
It sets up any public health official who has a discussion about models. There's a famous saying: "All models are wrong, but some are useful."
Just because the projections change, it's not proof of wrongness, it's not proof that the model is fatally flawed. In fact, I'd be really concerned if the projections didn't change based on new information. I would love it if this whole episode did lead to a better understanding of the scientific process and how scientific publishing fits into that — and doesn't fit into it.
Elisabeth
Yes, I'm with you. I'm very worried that the general audience's perspective is based on maybe watching too many movies where the scientist comes up with a conclusion one hour into the movie when everything is about to fail. Like that scene in Contagion where somebody injects, I think, eight monkeys, and one of the monkeys survives and boom we have the vaccine. That's not really how science works. Everything takes many, many years and many, many applications where usually your first ideas and your first hypothesis turn out to be completely wrong.
Then you go back to the drawing board, you develop another hypothesis and this is a very reiterative process that usually takes years. Most of the people who watch the movie might have a very wrong idea and wrong expectations about how science works. We're living in the movie Contagion and by September, we'll all be vaccinated and we can go on and live our lives. But that's not what is going to happen. It's going to take much, much longer and we're going to have to change the models every time and change our expectations. Just because we don't know all the numbers and all the facts yet.
Ivan
Generally it takes a fairly long time to change medical practice. A lot of times people see that as a bad thing. What I think that ignores, or at least doesn't take into as much account as I would, is that you don't want doctors and other health care professionals to turn on a dime and suddenly switch. Unless, of course, it turns out there was no evidence for what you were looking at.
It's a complicated situation.
Everybody wants scientists to be engineers, right?
Elisabeth
Right.
Ivan
I'm not saying engineering isn't scientific, nor am I saying that science is just completely whimsical, but there's a different process. It's a different way of looking at things and you can't just throw all the data into a big supercomputer, which is what I think a lot of people seem to want us to do, and then the obvious answer will come out on the other side.
Elisabeth
No. It's true and a lot of engineers suddenly feel their inherent need to solve this as a problem. They're not scientists and it's not building a bridge over a big river. But we're dealing with something that is very hard to solve because we don't understand the problem yet. I think scientists are usually first analyzing the problem and trying to understand what the problem actually is before you can even think about a solution.
Ivan
I think we're still at the understanding the problem phase.
Elisabeth
Exactly. And going back to the French group paper, that promised such a result and that was interpreted as such by a lot of people including presidents, but it's a very rare thing to find a medication that will have a 100% curation rate. That's something that I wish the people would understand better. We all want that to happen, but it's very unlikely and very unprecedented in the best of times.
Ivan
I would second that and also say that the world needs to better value the work that people like Elisabeth and others are doing. Because we're not going to get to a better answer if we're not rigorous about scrutinizing the literature and scrutinizing the methodology and scrutinizing the results.
"I quit my job to be able to do this work."
It's a relatively new phenomenon that you're able to do this at any scale at all, and even now it's at a very small scale. Elisabeth mentioned PubPeer and I'm a big fan — also full disclosure, I'm on their board of directors as a volunteer — it's a very powerful engine for readers and journal editors and other scientists to discuss issues.
And Elisabeth has used it really, really well. I think we need to start giving credit to people like that. And, also creating incentives for that kind of work in a way that science hasn't yet.
Elisabeth
Yeah. I quit my job to be able to do this work. It's really hard to combine it with a job either in academia or industry because we're looking for or criticizing papers and it's hard when you are still employed to do that.
I try to make it about the papers and do it in a polite way, but still it's a very hard job to do if you have a daytime job and a position and a career to worry about. Because if you're critical of other academics, that could actually mean the end of your career and that's sad. They should be more open to polite criticism.
Ivan
And for the general public, if you're reading a newspaper story or something online about a single study and it doesn't mention any other studies that have said the same thing or similar, or frankly, if it doesn't say anything about any studies that contradicted it, that's probably also telling you something.
Say you're looking at a huge painting of a shoreline, a beach, and a forest. Any single study is just a one-centimeter-by-one-centimeter square of any part of that canvas. If you just look at that, you would either think it was a painting of the sea, of a beach, or of the forest. It's actually all three of those things.
We just need to be patient, and that's very challenging to us as human beings, but we need to take the time to look at the whole picture.
DISCLAIMER: Neither Elisabeth Bik nor Ivan Oransky was compensated for participation in The Pandemic Issue. While the magazine's editors suggested broad topics for discussion, consistent with Bik's and Oransky's work, neither they nor the magazine's underwriters had any influence on their conversation.
[Editor's Note: This article was originally published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Want to Motivate Vaccinations? Message Optimism, Not Doom
There is a lot to be optimistic about regarding the new safe and highly effective vaccines, which are moving society closer toward the goal of close human contact once again.
After COVID-19 was declared a worldwide pandemic by the World Health Organization on March 11, 2020, life as we knew it altered dramatically and millions went into lockdown. Since then, most of the world has had to contend with masks, distancing, ventilation and cycles of lockdowns as surges flare up. Deaths from COVID-19 infection, along with economic and mental health effects from the shutdowns, have been devastating. The need for an ultimate solution -- safe and effective vaccines -- has been paramount.
On November 9, 2020 (just 8 months after the pandemic announcement), the press release for the first effective COVID-19 vaccine from Pfizer/BioNTech was issued, followed by positive announcements regarding the safety and efficacy of five other vaccines from Moderna, University of Oxford/AztraZeneca, Novavax, Johnson and Johnson and Sputnik V. The Moderna and Pfizer vaccines have earned emergency use authorization through the FDA in the United States and are being distributed. We -- after many long months -- are seeing control of the devastating COVID-19 pandemic glimmering into sight.
To be clear, these vaccine candidates for COVID-19, both authorized and not yet authorized, are highly effective and safe. In fact, across all trials and sites, all six vaccines were 100% effective in preventing hospitalizations and death from COVID-19.
All Vaccines' Phase 3 Clinical Data
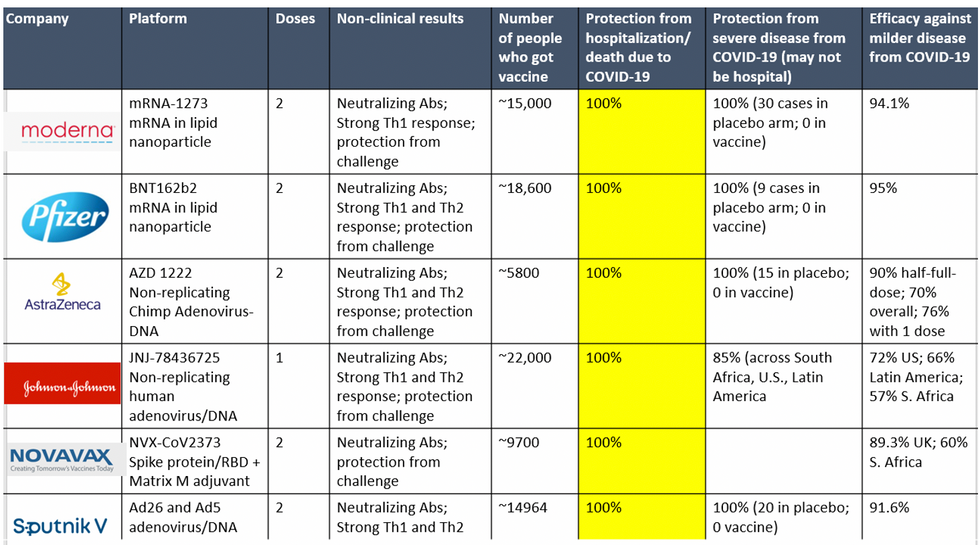
Complete protection against hospitalization and death from COVID-19 exhibited by all vaccines with phase 3 clinical trial data.
This astounding level of protection from SARS-CoV-2 from all vaccine candidates across multiple regions is likely due to robust T cell response from vaccination and will "defang" the virus from the concerns that led to COVID-19 restrictions initially: the ability of the virus to cause severe illness. This is a time of hope and optimism. After the devastating third surge of COVID-19 infections and deaths over the winter, we finally have an opportunity to stem the crisis – if only people readily accept the vaccines.
Amidst these incredible scientific advancements, however, public health officials and politicians have been pushing downright discouraging messaging. The ubiquitous talk of ongoing masks and distancing restrictions without any clear end in sight threatens to dampen uptake of the vaccines. It's imperative that we break down each concern and see if we can revitalize our public health messaging accordingly.
The first concern: we currently do not know if the vaccines block asymptomatic infection as well as symptomatic disease, since none of the phase 3 vaccine trials were set up to answer this question. However, there is biological plausibility that the antibodies and T-cell responses blocking symptomatic disease will also block asymptomatic infection in the nasal passages. IgG immunoglobulins (generated and measured by the vaccine trials) enter the nasal mucosa and systemic vaccinations generate IgA antibodies at mucosal surfaces. Monoclonal antibodies given to outpatients with COVID-19 hasten viral clearance from the airways.
Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other.
Moreover, data from the AztraZeneca trial (including in the phase 3 trial final results manuscript), where weekly self-swabbing was done by participants, and data from the Moderna trial, where a nasal swab was performed prior to the second dose, both showed risk reductions in asymptomatic infection with even a single dose. Finally, real-world data from a large Pfizer-based vaccine campaign in Israel shows a 50% reduction in infections (asymptomatic or symptomatic) after just the first dose.
Therefore, the likelihood of these vaccines blocking asymptomatic carriage, as well as symptomatic disease, is high. Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other. Moreover, as the percentage of vaccinated people increases, it will be increasingly untenable to impose restrictions on this group. Once herd immunity is reached, these restrictions can and should be abandoned altogether.
The second concern translating to "doom and gloom" messaging lately is around the identification of troubling new variants due to enhanced surveillance via viral sequencing. Four major variants circulating at this point (with others described in the past) are the B.1.1.7 variant ("UK variant"), B.1.351 ("South Africa variant), P.1. ("Brazil variant"), and the L452R variant identified in California. Although the UK variant is likely to be more transmissible, as is the South Africa variant, we have no reason to believe that masks, distancing and ventilation are ineffective against these variants.
Moreover, neutralizing antibody titers with the Pfizer and Moderna vaccines do not seem to be significantly reduced against the variants. Finally, although the Novavax 2-dose and Johnson and Johnson (J&J) 1-dose vaccines had lower rates of efficacy against moderate COVID-19 disease in South Africa, their efficacy against severe disease was impressively high. In fact J&J's vaccine still prevented 100% of hospitalizations and death from COVID-19. When combining both hospitalizations/deaths and severe symptoms managed at home, the J&J 1-dose vaccine was 85% protective across all three sites of the trial: the U.S., Latin America (including Brazil), and South Africa.
In South Africa, nearly all cases of COVID-19 (95%) were due to infection with the B.1.351 SARS-CoV-2 variant. Finally, since herd immunity does not rely on maximal immune responses among all individuals in a society, the Moderna/Pfizer/J&J vaccines are all likely to achieve that goal against variants. And thankfully, all of these vaccines can be easily modified to boost specifically against a new variant if needed (indeed, Moderna and Pfizer are already working on boosters against the prominent variants).
The third concern of some public health officials is that people will abandon all restrictions once vaccinated unless overly cautious messages are drilled into them. Indeed, the false idea that if you "give people an inch, they will take a mile" has been misinforming our messaging about mitigation since the beginning of the pandemic. For example, the very phrase "stay at home" with all of its non-applicability for essential workers and single individuals is stigmatizing and unrealistic for many. Instead, the message should have focused on how people can additively reduce their risks under different circumstances.
The public will be more inclined to trust health officials if those officials communicate with nuanced messages backed up by evidence, rather than with broad brushstrokes that shame. Therefore, we should be saying that "vaccinated people can be together with other vaccinated individuals without restrictions but must protect the unvaccinated with masks and distancing." And we can say "unvaccinated individuals should adhere to all current restrictions until vaccinated" without fear of misunderstandings. Indeed, this kind of layered advice has been communicated to people living with HIV and those without HIV for a long time (if you have HIV but partner does not, take these precautions; if both have HIV, you can do this, etc.).
Our heady progress in vaccine development, along with the incredible efficacy results of all of them, is unprecedented. However, we are at risk of undermining such progress if people balk at the vaccine because they don't believe it will make enough of a difference. One of the most critical messages we can deliver right now is that these vaccines will eventually free us from the restrictions of this pandemic. Let's use tiered messaging and clear communication to boost vaccine optimism and uptake, and get us to the goal of close human contact once again.
Inside Scoop: How a DARPA Scientist Helped Usher in a Game-Changing Covid Treatment
Amy Jenkins is a program manager for the Defense Advanced Research Projects Agency's Biological Technologies Office, which runs a project called the Pandemic Prevention Platform.
Amy Jenkins was in her office at DARPA, a research and development agency within the Department of Defense, when she first heard about a respiratory illness plaguing the Chinese city of Wuhan. Because she's a program manager for DARPA's Biological Technologies Office, her colleagues started stopping by. "It's really unusual, isn't it?" they would say.
At the time, China had a few dozen cases of what we now call COVID-19. "We should maybe keep an eye on that," she thought.
Early in 2020, still just keeping watch, she was visiting researchers working on DARPA's Pandemic Prevention Platform (P3), a project to develop treatments for "any known or previously unknown infectious threat," within 60 days of its appearance. "We looked at each other and said, 'Should we be doing something?'" she says.
For projects like P3, groups of scientists—often at universities and private companies—compete for DARPA contracts, and program managers like Jenkins oversee the work. Those that won the P3 bid included scientists at AbCellera Biologics, Inc., AstraZeneca, Duke University, and Vanderbilt University.
At the time Jenkins was talking to the P3 performers, though, they didn't have evidence of community transmission. "We would have to cross that bar before we considered doing anything," she says.
The world soon leapt far over that bar. By the time Jenkins and her team decided P3 should be doing something—with their real work beginning in late February--it was too late to prevent this pandemic. But she could help P3 dig into the chemical foundations of COVID-19's malfeasance, and cut off its roots. That work represents, in fact, her roots.
In late February 2020, DARPA received a single blood sample from a recovered COVID-19 patient, in which P3 researchers could go fishing for antibodies. The day it arrived, Jenkins's stomach roiled. "We get one shot," she thought.
Fighting the Smallest Enemies
Jenkins, who's in her early 40s, first got into germs the way many 90s kids did: by reading The Hot Zone, a novel about a hemorrhagic fever gone rogue. It wasn't exactly the disintegrating organs that hooked her. It was the idea that "these very pathogens that we can't even see can make us so sick and bring us to our knees," she says. Reading about scientists facing down deadly disease, she wondered, "How do these things make you so sick?"
She chased that question in college, majoring in both biomolecular science and chemistry, and later became an antibody expert. Antibodies are proteins that hook to a pathogen to block it from attaching to your cells, or tag it for destruction by the rest of the immune system. Soon, she jumped on the "monoclonal antibodies" train—developing synthetic versions of these natural defenses, which doctors can give to people to help them battle an early-stage infection, and even to prevent an infection from taking root after an exposure.
Jenkins likens the antibody treatments to the old aphorism about fishing: Vaccines teach your body how to fish, but antibodies simply give your body the pesca-fare. While that, as the saying goes, won't feed you for a lifetime, it will last a few weeks or months. Monoclonal antibodies thus are a promising preventative option in the immediate short-term when a vaccine hasn't yet been given (or hasn't had time to produce an immune response), as well as an important treatment weapon in the current fight. After former president Donald Trump contracted COVID-19, he received a monoclonal antibody treatment from biotech company Regeneron.
As for Jenkins, she started working as a DARPA Biological Technologies Office contractor soon after completing her postdoc. But it was a suit job, not a labcoat job. And suit jobs, at first, left Jenkins conflicted, worried about being bored. She'd give it a year, she thought. But the year expired, and bored she was not. Around five years later, in June 2019, the agency hired her to manage several of the office's programs. A year into that gig, the world was months into a pandemic.
The Pandemic Pivot
At DARPA, Jenkins inherited five programs, including P3. P3 works by taking blood from recovered people, fishing out their antibodies, identifying the most effective ones, and then figuring out how to manufacture them fast. Back then, P3 existed to help with nebulous, future outbreaks: Pandemic X. Not this pandemic. "I did not have a crystal ball," she says, "but I will say that all of us in the infectious diseases and public-health realm knew that the next pandemic was coming."
Three days after a January 2020 meeting with P3 researchers, COVID-19 appeared in Seattle, then began whipping through communities. The time had come for P3 teams to swivel. "We had done this," she says. "We had practiced this before." But would their methods stand up to something unknown, racing through the global population? "The big anxiety was, 'Wow, this was real,'" says Jenkins.
While facing down that realness, Jenkins was also managing other projects. In one called PREPARE, groups develop "medical countermeasures" that modulate a person's genetic code to boost their bodies' responses to threats. Another project, NOW, envisions shipping-container-sized factories that can make thousands of vaccine doses in days. And then there's Prometheus—which means "forethought" in Greek, and is the name of the god who stole fire and gave it to humans. Wrapping up as COVID ramped up, Prometheus aimed to identify people who are contagious—with whatever—before they start coughing, and even if they never do.
All of DARPA's projects focus on developing early-stage technology, passing it off to other agencies or industry to put it into operation. The orientation toward a specific goal appealed to Jenkins, as a contrast to academia. "You go down a rabbit hole for years at a time sometimes, chasing some concept you found interesting in the lab," she says. That's good for the human pursuit of knowledge, and leads to later applications, but DARPA wants a practical prototype—stat.
"Dual-Use" Technologies
That desire, though, and the fact that DARPA is a defense agency, present philosophical complications. "Bioethics in the national-security context turns all the dials up to 10+," says Jonathan Moreno, a medical ethicist at the University of Pennsylvania.
While developing antibody treatments to stem a pandemic seems straightforwardly good, all biological research—especially that backed by military money—requires evaluating potential knock-on applications, even those that might come from outside the entity that did the developing. As Moreno put it, "Albert Einstein wasn't thinking about blowing up Hiroshima." Particularly sensitive are so-called "dual-use" technologies—those tools that could be used for both benign and nefarious purposes, or are of interest to both the civilian and military worlds.
Moreno takes Prometheus itself as an example of "dual-use" technology. "Think about somebody wearing a suicide vest. Instead of a suicide vest, make them extremely contagious with something. The flu plus Ebola," he says. "Send them someplace, a sensitive environment. We would like to be able to defend against that"—not just tell whether Uncle Fred is bringing asymptomatic COVID home for Christmas. Prometheus, Jenkins says, had safety in mind from the get-go, and required contenders to "develop a risk mitigation plan" and "detail their strategy for appropriate control of information."
To look at a different program, if you can modulate genes to help healing, you probably know something (or know someone else could infer something) about how to hinder healing. Those sorts of risks are why PREPARE researchers got their own "ethical, legal, and social implications" panel, which meets quarterly "to ensure that we are performing all research and publications in a safe and ethical manner," says Jenkins.
DARPA as a whole, Moreno says, is institutionally sensitive to bioethics. The agency has ethics panels, and funded a 2014 National Academies assessment of how to address the "ethical, legal, and societal issues" around technology that has military relevance. "In the cases of biotechnologies where some of that research brushes up against what could legitimately be considered dual-use, that in itself justifies our investment," says Jenkins. "DARPA deliberately focuses on safety and countermeasures against potentially dangerous technologies, and we structure our programs to be transparent, safe, and legal."
Going Fishing
In late February 2020, DARPA received a single blood sample from a recovered COVID-19 patient, in which P3 researchers could go fishing for antibodies. The day it arrived, Jenkins's stomach roiled. "We get one shot," she thought.
As scientists from the P3-funded AbCellera went through the processes they'd practiced, Jenkins managed their work, tracking progress and relaying results. Soon, the team had isolated a suitable protein: bamlanivimab. It attaches to and blocks off the infamous spike proteins on SARS-CoV-2—those sticky suction-cups in illustrations. Partnering with Eli Lilly in a manufacturing agreement, the biotech company brought it to clinical trials in May, just a few months after its work on the deadly pathogen began, after much of the planet became a hot zone.
On November 10—Jenkins's favorite day at the (home) office—the FDA provided Eli Lilly emergency use authorization for bamlanivimab. But she's only mutedly screaming (with joy) inside her heart. "This pandemic isn't 'one morning we're going to wake up and it's all over,'" she says. When it is over, she and her colleagues plan to celebrate their promethean work. "I'm hoping to be able to do it in person," she says. "Until then, I have not taken a breath."

