The Sickest Babies Are Covered in Wires. New Tech Is Changing That.
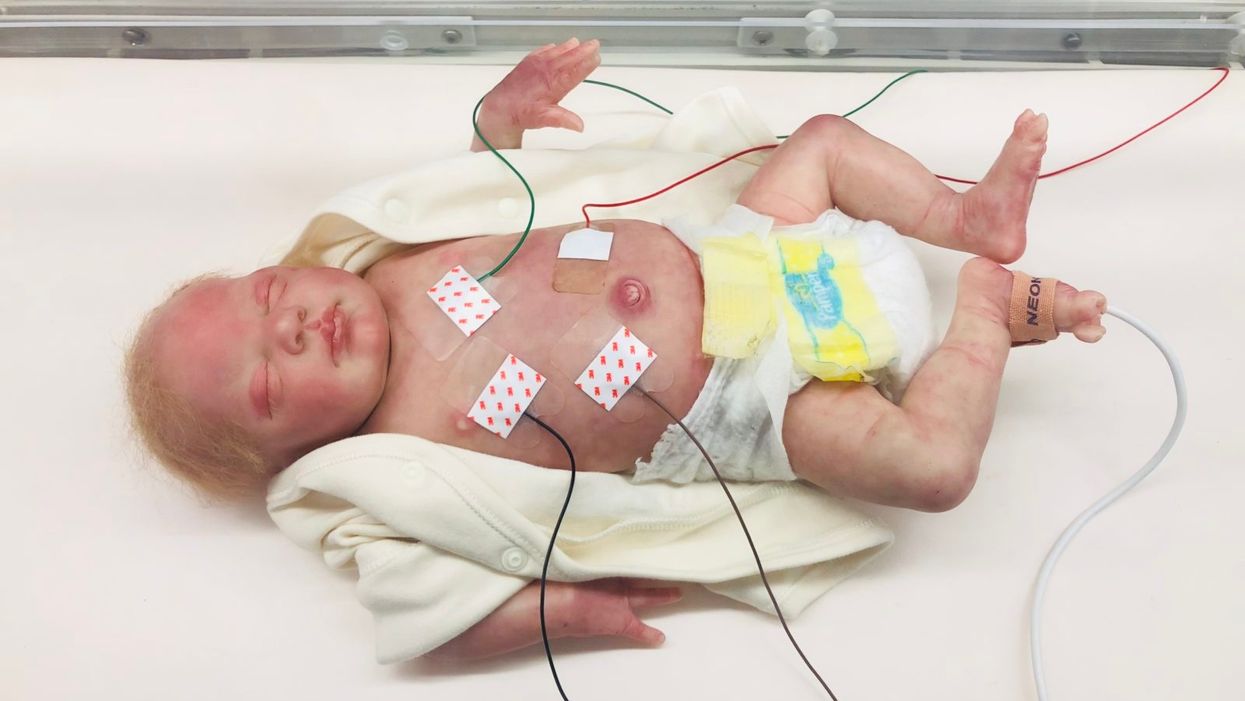
A wired baby in a neonatal intensive care unit.
I'll never forget the experience of having a child in the neonatal intensive care unit (NICU).
Now more than ever, we're working to remove the barriers between new parents and their infants.
It was another layer of uncertainty that filtered into my experience of being a first-time parent. There was so much I didn't know, and the wires attached to my son's small body for the first week of his life were a reminder of that.
I wanted to be the best mother possible. I deeply desired to bring my son home to start our lives. More than anything, I longed for a wireless baby whom I could hold and love freely without limitations.
The wires suggested my baby was fragile and it left me feeling severely unprepared, anxious, and depressed.
In recent years, research has documented the ways that NICU experiences take a toll on parents' mental health. But thankfully, medical technology is rapidly being developed to help reduce the emotional fallout of the NICU. Now more than ever, we're working to remove the barriers between new parents and their infants. The latest example is the first ever wireless monitoring system that was recently developed by a team at Northwestern University.
After listening to the needs of parents and medical staff, Debra Weese-Mayer, M.D., a professor of pediatric autonomic medicine at Feinberg School of Medicine, along with a team of materials scientists, engineers, dermatologists and pediatricians, set out to develop this potentially life-changing technology. Weese-Mayer believes wireless monitoring will have a significant impact for people on all sides of the NICU experience.
"With elimination of the cumbersome wires," she says, "the parents will find their infant more approachable/less intimidating and have improved access to their long-awaited but delivered-too-early infant, allowing them to begin skin-to-skin contact and holding with reduced concern for dislodging wires."
So how does the new system work?
Very thin "skin like" patches made of silicon rubber are placed on the surface of the skin to monitor vitals like heart rate, respiration rate, and body temperature. One patch is placed on the chest or back and the other is placed on the foot.
These patches are safer on the skin than previously used adhesives, reducing the cuts and infections associated with past methods. Finally, an antenna continuously delivers power, often from under the mattress.
The data collected from the patches stream from the body to a tablet or computer.

New wireless sensor technology is being studied to replace wired monitoring in NICUs in the coming years.
(Northwestern University)
Weese-Mayer hopes that wireless systems will be standard soon, but first they must undergo more thorough testing. "I would hope that in the next five years, wireless monitoring will be the standard in NICUs, but there are many essential validation steps before this technology will be embraced nationally," she says.
Until the new systems are ready, parents will be left struggling with the obstacles that wired monitoring presents.
Physical intimacy, for example, appears to have pain-reducing qualities -- something that is particularly important for babies who are battling serious illness. But wires make those cuddles more challenging.
There's also been minimal discussion about how wired monitoring can be particularly limiting for parents with disabilities and mobility aids, or even C-sections.
"When he was first born and I was recovering from my c-section, I couldn't deal with keeping the wires untangled while trying to sit down without hurting myself," says Rhiannon Giles, a writer from North Carolina, who delivered her son at just over 31 weeks after suffering from severe preeclampsia.
"The wires were awful," she remembers. "They fell off constantly when I shifted positions or he kicked a leg, which meant the monitors would alarm. It felt like an intrusion into the quiet little world I was trying to mentally create for us."
Over the last few years, researchers have begun to dive deeper into the literal and metaphorical challenges of wired monitoring.
For many parents, the wires prompt anxiety that worsens an already tense and vulnerable time.
I'll never forget the first time I got to hold my son without wires. It was the first time that motherhood felt manageable.
"Seeing my five-pound-babies covered in wires from head to toe rendered me completely overwhelmed," recalls Caila Smith, a mom of five from Indiana, whose NICU experience began when her twins were born pre-term. "The nurses seemed to handle them perfectly, but I was scared to touch them while they appeared so medically frail."
During the nine days it took for both twins to come home, the limited access she had to her babies started to impact her mental health. "If we would've had wireless sensors and monitors, it would've given us a much greater sense of freedom and confidence when snuggling our newborns," Smith says.
Besides enabling more natural interactions, wireless monitoring would make basic caregiving tasks much easier, like putting on a onesie.
"One thing I noticed is that many preemie outfits are made with zippers," points out Giles, "which just don't work well when your baby has wires coming off of them, head to toe."
Wired systems can pose issues for medical staff as well as parents.
"The main concern regarding wired systems is that they restrict access to the baby and often get tangled with other equipment, like IV lines," says Lamia Soghier, Medical Director of the Neonatal Intensive Care Unit at Children's National in Washington, D.C , who was also a NICU parent herself. "The nurses have to untangle the wires, which takes time, before handing the baby to the family."
I'll never forget the first time I got to hold my son without wires. It was the first time that motherhood felt manageable, and I couldn't stop myself from crying. Suddenly, anything felt possible and all the limitations from that first week of life seemed to fade away. The rise of wired-free monitoring will make some of the stressors that accompany NICU stays a thing of the past.
Podcast: Has the First 150-Year-Old Already Been Born
In today's podcast episode, Steven Austad explains why we should want to live a long time as long as that involves longer healthspans.
Steven Austad is a pioneer in the field of aging, with over 200 scientific papers and book chapters on pretty much every aspect of biological aging that you could think of. He’s also a strong believer in the potential for anti-aging therapies, and he puts his money where his mouth is. In 2001, he bet a billion dollars that the first person to reach 150-years-old had already been born. I had a chance to talk with Steven for today’s podcast and asked if he still thinks the bet was a good idea, since the oldest person so far (that we know of), Jeanne Calment, died back in 1997. A few days after our conversation, the oldest person in the world, Kane Tanaka, died at 119.
Steven is the Protective Life Endowed Chair in Health Aging Research, a Distinguished Professor and Chair of the Department of Biology at the University of Alabama Birmingham. He's also Senior Scientific Director of the American Federation for Aging Research, which is managing a groundbreaking longevity research trial that started this year. Steven is also a great science communicator with five books, including one that comes out later this year, Methuselah’s Zoo, and he publishes prolifically in national media outlets.
See the rest of his bio linked below in the show notes.
Listen to the Episode
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google

Steven Austad is featured in the latest episode of Making Sense of Science. He's a distinguished professor of biology at the University of Alabama Birmingham and has a new book due to be published in August, Methuselah's Zoo.
Photo by Steve Wood
Show notes:
2:36 - Steven explains why a particular opossum convinced him to dedicate his career to studying longevity.
6:48 - Steven's billion dollar bet that someone alive today will make it to 150-years-old.
9:15 - The most likely people to make it to 150 (Hint: not men).
10:38 - I ask Steven about Elon Musk’s comments this month that if people lived a really long time, “we’d be stuck with old ideas and society wouldn’t advance.” Steve isn’t so fond of that take.
13:34 - Why women are winning maybe the most important battle of sexes: staying alive. This is an area that Steven has led research on (see show notes).
18:20 - Why women, on average, actually have more morbidities earlier than men, even though they live longer.
23:10 - How the pandemic could affect sex differences in longevity.
24:55 - How often should people work out and get other physical activity to maximize longevity and health span?
29:09 - Steven gave me the latest update on the TAME trial on metformin, and how he and others longevity experts designed this groundbreaking research on longevity not in their offices, not on a zoom call, but in a castle in the Spanish countryside.
32:10 - Which anti-aging therapies are the most promising at this point for future research.
39:32 - The drug cocktail approach to address multiple hallmarks of aging.
41:00 - How to read health news like a scientist.
45:38 - Should we try a Manhattan project for aging?
48:47 - Can Jeff Bezos and Larry Ellison help us live to 150?
Show links:
Steven Austad's bio
Pre-order Steven's new book, Methuselah's Zoo - https://www.amazon.com/dp/B09M2QGRJR/ref=dp-kindle-redirect?_encoding=UTF8&btkr=1
Steven's journal article on Sex Differences in Lifespan - https://pubmed.ncbi.nlm.nih.gov/27304504/
Elon Musk's comments on super longevity "asphyxiating" society - https://www.cnbc.com/2022/04/11/elon-musk-on-avoid...
Steven's article on how to read news articles about health like a pro - https://www.nextavenue.org/how-to-read-health-news...
AFAR's research on Targeting Aging with Metformin (TAME) - https://www.afar.org/tame-trial
New therapy may improve stem cell transplants for blood cancers
Ivan Dimov, Jeroen Bekaert and Nate Fernhoff - pictured here - recognized the need for a more effective cell sorting technology to reduce the risk of Graft vs Host disease, which affects many cancer patients after receiving stem cell transplants.
In 2018, Robyn was diagnosed with myelofibrosis, a blood cancer causing chronic inflammation and scarring. As a research scientist by training, she knew she had limited options. A stem cell transplant is a terminally ill patient's best chance for survival against blood cancers, including leukaemia. It works by destroying a patient's cancer cells and replacing them with healthy cells from a donor.
However, there is a huge risk of Graft vs Host disease (GVHD), which affects around 30-40% of recipients. Patients receive billions of cells in a stem cell transplant but only a fraction are beneficial. The rest can attack healthy tissue leading to GVHD. It affects the skin, gut and lungs and can be truly debilitating.
Currently, steroids are used to try and prevent GVHD, but they have many side effects and are effective in only 50% of cases. “I spoke with my doctors and reached out to patients managing GVHD,” says Robyn, who prefers not to use her last name for privacy reasons. “My concerns really escalated for what I might face post-transplant.”
Then she heard about a new highly precise cell therapy developed by a company called Orca Bio, which gives patients more beneficial cells and fewer cells that cause GVHD. She decided to take part in their phase 2 trial.
How It Works
In stem cell transplants, patients receive immune cells and stem cells. The donor immune cells or T cells attack and kill malignant cells. This is the graft vs leukaemia effect (GVL). The stem cells generate new healthy cells.
Unfortunately, T cells can also cause GVHD, but a rare subset of T cells, called T regulatory cells, can actually prevent GVHD.
Orca’s cell sorting technology distinguishes T regulatory cells from stem cells and conventional T cells on a large scale. It’s this cell sorting technology which has enabled them to create their new cell therapy, called Orca T. It contains a precise combination of stem cells and immune cells with more T regulatory cells and fewer conventional T cells than in a typical stem cell transplant.
“Ivan Dimov’s idea was to spread out the cells, keep them stationary and then use laser scanning to sort the cells,” explains Nate Fernhoff, co-founder of Orca Bio. “The beauty here is that lasers don't care how quickly you move them.”
Over the past 40 years, scientists have been trying to create stem cell grafts that contain the beneficial cells whilst removing the cells that cause GVHD. What makes it even harder is that most transplant centers aren’t able to manipulate grafts to create a precise combination of cells.
Innovative Cell Sorting
Ivan Dimov, Jeroen Bekaert and Nate Fernhoff came up with the idea behind Orca as postdocs at Stanford, working with cell pioneer Irving Weissman. They recognised the need for a more effective cell sorting technology. In a small study at Stanford, Professor Robert Negrin had discovered a combination of T cells, T regulatory cells and stem cells which prevented GVHD but retained the beneficial graft vs leukaemia effect (GVL). However, manufacturing was problematic. Conventional cell sorting is extremely slow and specific. Negrin was only able to make seven highly precise products, for seven patients, in a year. Annual worldwide cases of blood cancer number over 1.2 million.
“We started Orca with this idea: how do we use manufacturing solutions to impact cell therapies,” co-founder Fernhoff reveals. In conventional cell sorting, cells move past a stationary laser which analyses each cell. But cells can only be moved so quickly. At a certain point they start to experience stress and break down. This makes it very difficult to sort the 100 billion cells from a donor in a stem cell transplant.
“Ivan Dimov’s idea was to spread out the cells, keep them stationary and then use laser scanning to sort the cells,” Fernhoff explains. “The beauty here is that lasers don't care how quickly you move them.” They developed this technology and called it Orca Sort. It enabled Orca to make up to six products per week in the first year of manufacturing.
Every product Orca makes is for one patient. The donor is uniquely matched to the patient. They have to carry out the cell sorting procedure each time. Everything also has to be done extremely quickly. They infuse fresh living cells from the donor's vein to the patient's within 72 hours.
“We’ve treated almost 200 patients in all the Orca trials, and you can't do that if you don't fix the manufacturing process,” Fernhoff says. “We're working on what we think is an incredibly promising drug, but it's all been enabled by figuring out how to make a high precision cell therapy at scale.”
Clinical Trials
Orca revealed the results of their phase 1b and phase 2 trials at the end of last year. In their phase 2 trial only 3% of the 29 patients treated with Orca T cell therapy developed chronic GVHD in the first year after treatment. Comparatively, 43% of the 95 patients given a conventional stem cell transplant in a contemporary Stanford trial developed chronic GVHD. Of the 109 patients tested in phase 1b and phase 2 trials, 74% using Orca T didn't relapse or develop any form of GVHD compared to 34% in the control trial.
“Until a randomised study is done, we can make no assumption about the relative efficacy of this approach," says Jeff Szer, professor of haematology at the Royal Melbourne Hospital. "But the holy grail of separating GVHD and GVL is still there and this is a step towards realising that dream.”
Stan Riddell, an immunology professor, at Fred Hutchinson Cancer Centre, believes Orca T is highly promising. “Orca has advanced cell selection processes with innovative methodology and can engineer grafts with greater precision to add cell subsets that may further contribute to beneficial outcomes,” he says. “Their results in phase 1 and phase 2 studies are very exciting and offer the potential of providing a new standard of care for stem cell transplant.”
However, though it is an “intriguing step,” there’s a need for further testing, according to Jeff Szer, a professor of haematology at the Peter MacCallum Cancer Centre at the Royal Melbourne Hospital.
“The numbers tested were tiny and comparing the outcomes to anything from a phase 1/2 setting is risky,” says Szer. “Until a randomised study is done, we can make no assumption about the relative efficacy of this approach. But the holy grail of separating GVHD and GVL is still there and this is a step towards realising that dream.”
The Future
The team is soon starting Phase 3 trials for Orca T. Its previous success has led them to develop Orca Q, a cell therapy for patients who can't find an exact donor match. Transplants for patients who are only a half-match or mismatched are not widely used because there is a greater risk of GVHD. Orca Q has the potential to control GVHD even more and improve access to transplants for many patients.
Fernhoff hopes they’ll be able to help people not just with blood cancers but also with other blood and immune disorders. If a patient has a debilitating disease which isn't life threatening, the risk of GVHD outweighs the potential benefits of a stem cell transplant. The Orca products could take away that risk.
Meanwhile, Robyn has no regrets about participating in the Phase 2 trial. “It was a serious decision to make but I'm forever grateful that I did,” she says. “I have resumed a quality of life aligned with how I felt pre-transplant. I have not had a single issue with GVHD.”
“I want to be able to get one of these products to every patient who could benefit from it,” Fernhoff says. “It's really exciting to think about how Orca's products could be applied to all sorts of autoimmune disorders.”

