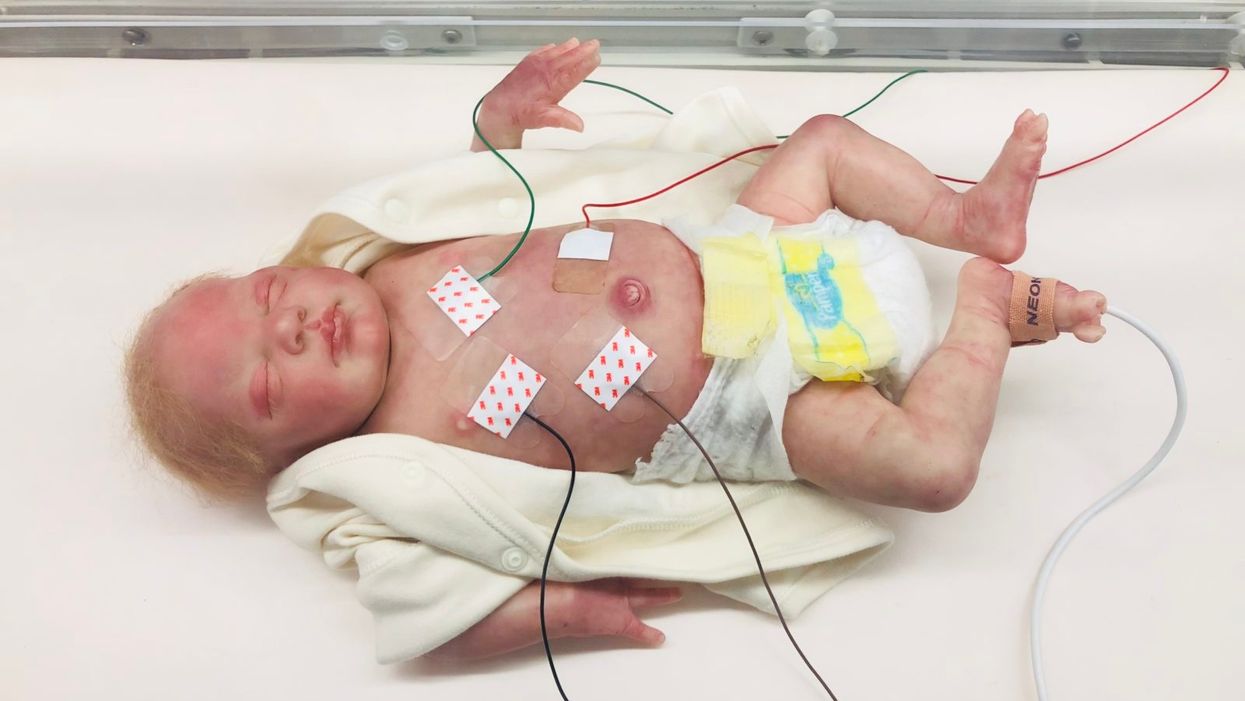
A wired baby in a neonatal intensive care unit.
I'll never forget the experience of having a child in the neonatal intensive care unit (NICU).
Now more than ever, we're working to remove the barriers between new parents and their infants.
It was another layer of uncertainty that filtered into my experience of being a first-time parent. There was so much I didn't know, and the wires attached to my son's small body for the first week of his life were a reminder of that.
I wanted to be the best mother possible. I deeply desired to bring my son home to start our lives. More than anything, I longed for a wireless baby whom I could hold and love freely without limitations.
The wires suggested my baby was fragile and it left me feeling severely unprepared, anxious, and depressed.
In recent years, research has documented the ways that NICU experiences take a toll on parents' mental health. But thankfully, medical technology is rapidly being developed to help reduce the emotional fallout of the NICU. Now more than ever, we're working to remove the barriers between new parents and their infants. The latest example is the first ever wireless monitoring system that was recently developed by a team at Northwestern University.
After listening to the needs of parents and medical staff, Debra Weese-Mayer, M.D., a professor of pediatric autonomic medicine at Feinberg School of Medicine, along with a team of materials scientists, engineers, dermatologists and pediatricians, set out to develop this potentially life-changing technology. Weese-Mayer believes wireless monitoring will have a significant impact for people on all sides of the NICU experience.
"With elimination of the cumbersome wires," she says, "the parents will find their infant more approachable/less intimidating and have improved access to their long-awaited but delivered-too-early infant, allowing them to begin skin-to-skin contact and holding with reduced concern for dislodging wires."
So how does the new system work?
Very thin "skin like" patches made of silicon rubber are placed on the surface of the skin to monitor vitals like heart rate, respiration rate, and body temperature. One patch is placed on the chest or back and the other is placed on the foot.
These patches are safer on the skin than previously used adhesives, reducing the cuts and infections associated with past methods. Finally, an antenna continuously delivers power, often from under the mattress.
The data collected from the patches stream from the body to a tablet or computer.

New wireless sensor technology is being studied to replace wired monitoring in NICUs in the coming years.
(Northwestern University)
Weese-Mayer hopes that wireless systems will be standard soon, but first they must undergo more thorough testing. "I would hope that in the next five years, wireless monitoring will be the standard in NICUs, but there are many essential validation steps before this technology will be embraced nationally," she says.
Until the new systems are ready, parents will be left struggling with the obstacles that wired monitoring presents.
Physical intimacy, for example, appears to have pain-reducing qualities -- something that is particularly important for babies who are battling serious illness. But wires make those cuddles more challenging.
There's also been minimal discussion about how wired monitoring can be particularly limiting for parents with disabilities and mobility aids, or even C-sections.
"When he was first born and I was recovering from my c-section, I couldn't deal with keeping the wires untangled while trying to sit down without hurting myself," says Rhiannon Giles, a writer from North Carolina, who delivered her son at just over 31 weeks after suffering from severe preeclampsia.
"The wires were awful," she remembers. "They fell off constantly when I shifted positions or he kicked a leg, which meant the monitors would alarm. It felt like an intrusion into the quiet little world I was trying to mentally create for us."
Over the last few years, researchers have begun to dive deeper into the literal and metaphorical challenges of wired monitoring.
For many parents, the wires prompt anxiety that worsens an already tense and vulnerable time.
I'll never forget the first time I got to hold my son without wires. It was the first time that motherhood felt manageable.
"Seeing my five-pound-babies covered in wires from head to toe rendered me completely overwhelmed," recalls Caila Smith, a mom of five from Indiana, whose NICU experience began when her twins were born pre-term. "The nurses seemed to handle them perfectly, but I was scared to touch them while they appeared so medically frail."
During the nine days it took for both twins to come home, the limited access she had to her babies started to impact her mental health. "If we would've had wireless sensors and monitors, it would've given us a much greater sense of freedom and confidence when snuggling our newborns," Smith says.
Besides enabling more natural interactions, wireless monitoring would make basic caregiving tasks much easier, like putting on a onesie.
"One thing I noticed is that many preemie outfits are made with zippers," points out Giles, "which just don't work well when your baby has wires coming off of them, head to toe."
Wired systems can pose issues for medical staff as well as parents.
"The main concern regarding wired systems is that they restrict access to the baby and often get tangled with other equipment, like IV lines," says Lamia Soghier, Medical Director of the Neonatal Intensive Care Unit at Children's National in Washington, D.C , who was also a NICU parent herself. "The nurses have to untangle the wires, which takes time, before handing the baby to the family."
I'll never forget the first time I got to hold my son without wires. It was the first time that motherhood felt manageable, and I couldn't stop myself from crying. Suddenly, anything felt possible and all the limitations from that first week of life seemed to fade away. The rise of wired-free monitoring will make some of the stressors that accompany NICU stays a thing of the past.
Black Participants Are Sorely Absent from Medical Research
Black participants are under-represented in clinical research.
After years of suffering from mysterious symptoms, my mother Janice Thomas finally found a doctor who correctly diagnosed her with two autoimmune diseases, Lupus and Sjogren's. Both diseases are more prevalent in the black population than in other races and are often misdiagnosed.
The National Institutes of Health has found that minorities make up less than 10 percent of trial participants.
Like many chronic health conditions, a lack of understanding persists about their causes, individual manifestations, and best treatment strategies.
On the search for relief from chronic pain, my mother started researching options and decided to participate in clinical trials as a way to gain much-needed insights. In return, she received discounted medical testing and has played an active role in finding answers for all.
"When my doctor told me I could get financial or medical benefits from participating in clinical trials for the same test I was already doing, I figured it would be an easy way to get some answers at little to no cost," she says.
As a person of color, her presence in clinical studies is rare. The National Institutes of Health has found that minorities make up less than 10 percent of trial participants.
Without trial participation that is reflective of the general population, pharmaceutical companies and medical professionals are left guessing how various drugs work across racial lines. For example, albuterol, a widely used asthma treatment, was found to have decreased effectiveness for black American and Puerto Rican children. Many high mortality conditions, like cancer, also show different outcomes based on race.
Over the last decade, the pervasive lack of representation has left communities of color demanding higher levels of involvement in the research process. However, no consensus yet exists on how best to achieve this.
But experts suggest that before we can improve black participation in medical studies, key misconceptions must be addressed, such as the false assumption that such patients are unwilling to participate because they distrust scientists.
Jill A. Fisher, a professor in the Center for Bioethics at the University of North Carolina at Chapel Hill, learned in one study that mistrust wasn't the main barrier for black Americans. "There is a lot of evidence that researchers' recruitment of black Americans is generally poorly done, with many black patients simply not asked," Fisher says. "Moreover, the underrepresentation of black Americans is primarily true for efficacy trials - those testing whether an investigational drug might therapeutically benefit patients with specific illnesses."
Without increased minority participation, research will not accurately reflect the diversity of the general population.
Dr. Joyce Balls-Berry, a psychiatric epidemiologist and health educator, agrees that black Americans are often overlooked in the process. One study she conducted found that "enrollment of minorities in clinical trials meant using a variety of culturally appropriate strategies to engage participants," she explained.
To overcome this hurdle, The National Black Church Initiative (NBCI), a faith-based organization made up of 34,000 churches and over 15.7 million African Americans, last year urged the Food and Drug Administration to mandate diversity in all clinical trials before approving a drug or device. However, the FDA declined to implement the mandate, declaring that they don't have the authority to regulate diversity in clinical trials.
"African Americans have not been successfully incorporated into the advancement of medicine and research technologies as legitimate and natural born citizens of this country," admonishes NBCI's president Rev. Anthony Evans.
His words conjure a reminder of the medical system's insidious history for people of color. The most infamous incident is the Tuskegee syphilis scandal, in which white government doctors perpetrated harmful experiments on hundreds of unsuspecting black men for forty years, until the research was shut down in the early 1970s.
Today, in the second decade of twenty-first century, the pernicious narrative that blacks are outsiders in science and medicine must be challenged, says Dr. Danielle N. Lee, assistant professor of biological sciences at Southern Illinois University. And having majority white participants in clinical trials only furthers the notion that "whiteness" is the default.
According to Lee, black individuals often see themselves disconnected from scientific and medical processes. "One of the critiques with science and medical research is that communities of color, and black communities in particular, regard ourselves as outsiders of science," Lee says. "We are othered."
Without increased minority participation, research will not accurately reflect the diversity of the general population.
"We are all human, but we are different, and yes, even different populations of people require modified medical responses," she points out.
Another obstacle is that many trials have health requirements that exclude black Americans, like not wanting individuals who have high blood pressure or a history of stroke. Considering that this group faces health disparities at a higher rate than whites, this eliminates eligibility for millions of potential participants.
One way to increase the diversity in sample participation without an FDA mandate is to include more black Americans in both volunteer and clinical roles during the research process to increase accountability in treatment, education, and advocacy.
"When more of us participate in clinical trials, we help build out the basic data sets that account for health disparities from the start, not after the fact," Lee says. She also suggests that researchers involve black patient representatives throughout the clinical trial process, from the study design to the interpretation of results.
"This allows for the black community to give insight on how to increase trial enrollment and help reduce stigma," she explains.
Thankfully, partnerships are popping up like the one between The Howard University's Cancer Center and Driver, a platform that connects cancer patients to treatment and trials. These sorts of targeted and culturally tailored efforts allow black patients to receive assistance from well-respected organizations.
Some observers suggest that the federal government and pharmaceutical industries must step up to address the gap.
However, some experts say that the black community should not be held solely responsible for solving a problem it did not cause. Instead, some observers suggest that the federal government and pharmaceutical industries must step up to address the gap.
According to Balls-Berry, socioeconomic barriers like transportation, time off work, and childcare related to trial participation must be removed. "These are real-world issues and yet many times researchers have not included these things in their budgets."
When asked to comment, a spokesperson for BIO, the world's largest biotech trade association, emailed the following statement:
"BIO believes that that our members' products and services should address the needs of a diverse population, and enhancing participation in clinical trials by a diverse patient population is a priority for BIO and our member companies. By investing in patient education to improve awareness of clinical trial opportunities, we can reduce disparities in clinical research to better reflect the country's changing demographics."
For my mother, the patient suffering from autoimmune disease, the need for broad participation in medical research is clear. "Without clinical trials, we would have less diagnosis and solutions to diseases," she says. "I think it's an underutilized resource."