The Sickest Babies Are Covered in Wires. New Tech Is Changing That.
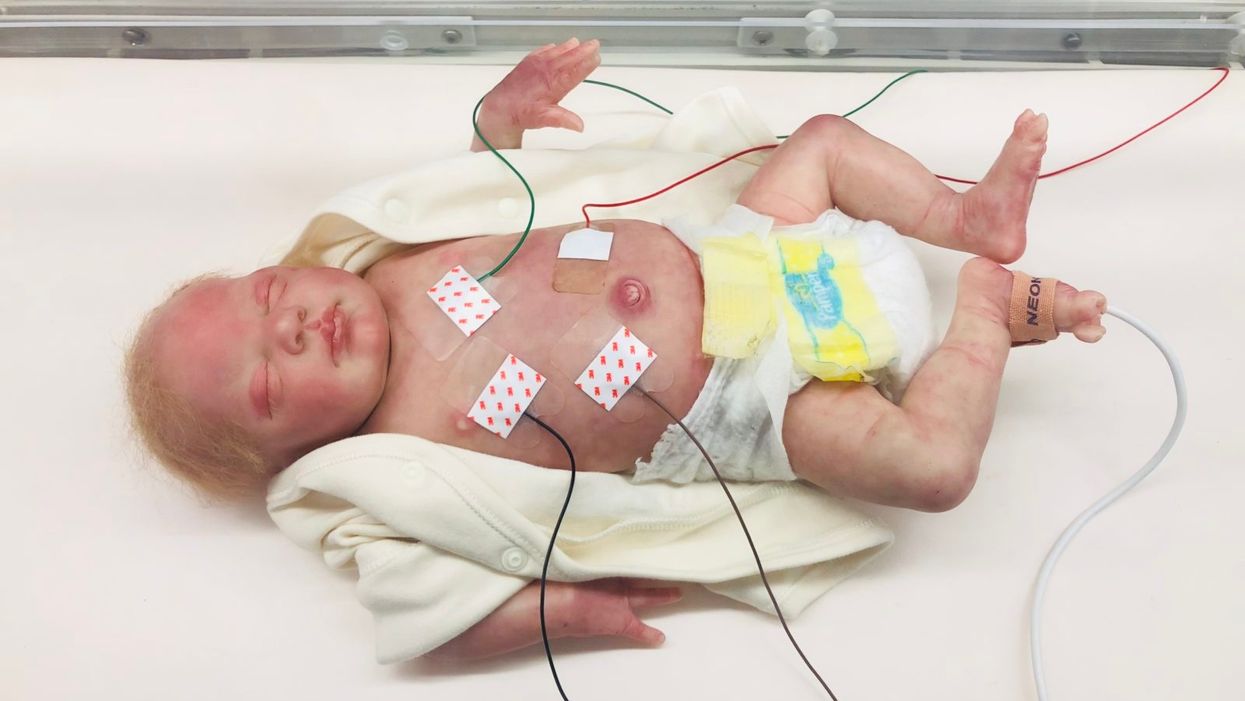
A wired baby in a neonatal intensive care unit.
I'll never forget the experience of having a child in the neonatal intensive care unit (NICU).
Now more than ever, we're working to remove the barriers between new parents and their infants.
It was another layer of uncertainty that filtered into my experience of being a first-time parent. There was so much I didn't know, and the wires attached to my son's small body for the first week of his life were a reminder of that.
I wanted to be the best mother possible. I deeply desired to bring my son home to start our lives. More than anything, I longed for a wireless baby whom I could hold and love freely without limitations.
The wires suggested my baby was fragile and it left me feeling severely unprepared, anxious, and depressed.
In recent years, research has documented the ways that NICU experiences take a toll on parents' mental health. But thankfully, medical technology is rapidly being developed to help reduce the emotional fallout of the NICU. Now more than ever, we're working to remove the barriers between new parents and their infants. The latest example is the first ever wireless monitoring system that was recently developed by a team at Northwestern University.
After listening to the needs of parents and medical staff, Debra Weese-Mayer, M.D., a professor of pediatric autonomic medicine at Feinberg School of Medicine, along with a team of materials scientists, engineers, dermatologists and pediatricians, set out to develop this potentially life-changing technology. Weese-Mayer believes wireless monitoring will have a significant impact for people on all sides of the NICU experience.
"With elimination of the cumbersome wires," she says, "the parents will find their infant more approachable/less intimidating and have improved access to their long-awaited but delivered-too-early infant, allowing them to begin skin-to-skin contact and holding with reduced concern for dislodging wires."
So how does the new system work?
Very thin "skin like" patches made of silicon rubber are placed on the surface of the skin to monitor vitals like heart rate, respiration rate, and body temperature. One patch is placed on the chest or back and the other is placed on the foot.
These patches are safer on the skin than previously used adhesives, reducing the cuts and infections associated with past methods. Finally, an antenna continuously delivers power, often from under the mattress.
The data collected from the patches stream from the body to a tablet or computer.

New wireless sensor technology is being studied to replace wired monitoring in NICUs in the coming years.
(Northwestern University)
Weese-Mayer hopes that wireless systems will be standard soon, but first they must undergo more thorough testing. "I would hope that in the next five years, wireless monitoring will be the standard in NICUs, but there are many essential validation steps before this technology will be embraced nationally," she says.
Until the new systems are ready, parents will be left struggling with the obstacles that wired monitoring presents.
Physical intimacy, for example, appears to have pain-reducing qualities -- something that is particularly important for babies who are battling serious illness. But wires make those cuddles more challenging.
There's also been minimal discussion about how wired monitoring can be particularly limiting for parents with disabilities and mobility aids, or even C-sections.
"When he was first born and I was recovering from my c-section, I couldn't deal with keeping the wires untangled while trying to sit down without hurting myself," says Rhiannon Giles, a writer from North Carolina, who delivered her son at just over 31 weeks after suffering from severe preeclampsia.
"The wires were awful," she remembers. "They fell off constantly when I shifted positions or he kicked a leg, which meant the monitors would alarm. It felt like an intrusion into the quiet little world I was trying to mentally create for us."
Over the last few years, researchers have begun to dive deeper into the literal and metaphorical challenges of wired monitoring.
For many parents, the wires prompt anxiety that worsens an already tense and vulnerable time.
I'll never forget the first time I got to hold my son without wires. It was the first time that motherhood felt manageable.
"Seeing my five-pound-babies covered in wires from head to toe rendered me completely overwhelmed," recalls Caila Smith, a mom of five from Indiana, whose NICU experience began when her twins were born pre-term. "The nurses seemed to handle them perfectly, but I was scared to touch them while they appeared so medically frail."
During the nine days it took for both twins to come home, the limited access she had to her babies started to impact her mental health. "If we would've had wireless sensors and monitors, it would've given us a much greater sense of freedom and confidence when snuggling our newborns," Smith says.
Besides enabling more natural interactions, wireless monitoring would make basic caregiving tasks much easier, like putting on a onesie.
"One thing I noticed is that many preemie outfits are made with zippers," points out Giles, "which just don't work well when your baby has wires coming off of them, head to toe."
Wired systems can pose issues for medical staff as well as parents.
"The main concern regarding wired systems is that they restrict access to the baby and often get tangled with other equipment, like IV lines," says Lamia Soghier, Medical Director of the Neonatal Intensive Care Unit at Children's National in Washington, D.C , who was also a NICU parent herself. "The nurses have to untangle the wires, which takes time, before handing the baby to the family."
I'll never forget the first time I got to hold my son without wires. It was the first time that motherhood felt manageable, and I couldn't stop myself from crying. Suddenly, anything felt possible and all the limitations from that first week of life seemed to fade away. The rise of wired-free monitoring will make some of the stressors that accompany NICU stays a thing of the past.
New Tech Can Predict Breast Cancer Years in Advance
Breast cancer survivors rally in a support group.
Every two minutes, a woman is diagnosed with breast cancer. The question is, can those at high risk be identified early enough to survive?
New AI software has predicted risk equally well in both white and black women for the first time.
The current standard practice in medicine is not exactly precise. It relies on age, family history of cancer, and breast density, among other factors, to determine risk. But these factors do not always tell the whole story, leaving many women to slip through the cracks. In addition, a racial gap persists in breast cancer treatment and survival. African-American women are 42 percent more likely to die from the disease despite relatively equal rates of diagnosis.
But now those grim statistics could be changing. A team of researchers from MIT's Computer Science and Artificial Intelligence Laboratory have developed a deep learning model that can more accurately predict a patient's breast cancer risk compared to established clinical guidelines – and it has predicted risk equally well in both white and black women for the first time.
The Lowdown
Study results published in Radiology described how the AI software read mammogram images from more than 60,000 patients at Massachusetts General Hospital to identify subtle differences in breast tissue that pointed to potential risk factors, even in their earliest stages. The team accessed the patients' actual diagnoses and determined that the AI model was able to correctly place 31 percent of all cancer patients in the highest-risk category of developing breast cancer within five years of the examination, compared to just 18 percent for existing models.
"Each image has hundreds of thousands of pixels identifying something that may not necessarily be detected by the human eye," said MIT professor Regina Barzilay, one of the study's lead authors. "We all have limited visual capacities so it seems some machines trained on hundreds of thousands of images with a known outcome can capture correlations the human eye might not notice."
Barzilay, a breast cancer survivor herself, had abnormal tissue patterns on mammograms in 2012 and 2013, but wasn't diagnosed until after a 2014 image reading, illustrating the limitations of human processing alone.

MIT professor Regina Barzilay, a lead author on the new study and a breast cancer survivor herself.
(Courtesy MIT)
Next up: The MIT team is looking at training the model to detect other cancers and health risks. Barzilay recalls how a cardiologist told her during a conference that women with heart diseases had a different pattern of calcification on their mammograms, demonstrating how already existing images can be used to extract other pieces of information about a person's health status.
Integration of the AI model in standard care could help doctors better tailor screening and prevention programs based on actual instead of perceived risk. Patients who might register as higher risk by current guidelines could be identified as lower risk, helping resolve conflicting opinions about how early and how often women should receive mammograms.
Open Questions: While the results were promising, it's unknown how well the model will work on a larger scale, as the study looked at data from just one institution and used mammograms supplied by just one hospital. Some risk factor information was also unavailable for certain patients during the study, leaving researchers unable to fully compare the AI model's performance to that of the traditional standard.
One incentive to wider implementation and study, however, is the bonus that no new hardware is required to use the AI model. With other institutions now showing interest, this software could lead to earlier routine detection and treatment of breast cancer — resulting in more lives saved.
A pregnant woman is uncertain which medicine is safe to take.
Sarah Mancoll was 22 years old when she noticed a bald spot on the back of her head. A dermatologist confirmed that it was alopecia aerata, an autoimmune disorder that causes hair loss.
Of 213 new drugs approved from 2003 to 2012, only five percent included any data from pregnant women.
She successfully treated the condition with corticosteroid shots for nearly 10 years. Then Mancoll and her husband began thinking about starting a family. Would the shots be safe for her while pregnant? For the fetus? What about breastfeeding?
Mancoll consulted her primary care physician, her dermatologist, even a pediatrician. Without clinical data, no one could give her a definitive answer, so she stopped treatment to be "on the safe side." By the time her son was born, she'd lost at least half her hair. She returned to her Washington, D.C., public policy job two months later entirely bald—and without either eyebrows or eyelashes.
After having two more children in quick succession, Mancoll recently resumed the shots but didn't forget her experience. Today, she is an advocate for including more pregnant and lactating women in clinical studies so they can have more information about therapies than she did.
"I live a very privileged life, and I'll do just fine with or without hair, but it's not just about me," Mancoll said. "It's about a huge population of women who are being disenfranchised…They're invisible."
About 4 million women give birth each year in the United States, and many face medical conditions, from hypertension and diabetes to psychiatric disorders. A 2011 study showed that most women reported taking at least one medication while pregnant between 1976 and 2008. But for decades, pregnant and lactating women have been largely excluded from clinical drug studies that rigorously test medications for safety and effectiveness.
An estimated 98 percent of government-approved drug treatments between 2000 and 2010 had insufficient data to determine risk to the fetus, and close to 75 percent had no human pregnancy data at all. All told, of 213 new pharmaceuticals approved from 2003 to 2012, only five percent included any data from pregnant women.
But recent developments suggest that could be changing. Amid widespread concerns about increased maternal mortality rates, women's health advocates, physicians, and researchers are sensing and encouraging a cultural shift toward protecting women through responsible research instead of from research.
"The question is not whether to do research with pregnant women, but how," Anne Drapkin Lyerly, professor and associate director of the Center for Bioethics at the University of North Carolina at Chapel Hill, wrote last year in an op-ed. "These advances are essential. It is well past time—and it is morally imperative—for research to benefit pregnant women."
"In excluding pregnant women from drug trials to protect them from experimentation, we subject them to uncontrolled experimentation."
To that end, the American College of Obstetricians and Gynecologists' Committee on Ethics acknowledged that research trials need to be better designed so they don't "inappropriately constrain the reproductive choices of study participants or unnecessarily exclude pregnant women." A federal task force also called for significantly expanded research and the removal of regulatory barriers that make it difficult for pregnant and lactating women to participate in research.
Several months ago, a government change to a regulation known as the Common Rule took effect, removing pregnant women as a "vulnerable population" in need of special protections -- a designation that had made it more difficult to enroll them in clinical drug studies. And just last week, the U.S. Food and Drug Administration (FDA) issued new draft guidances for industry on when and how to include pregnant and lactating women in clinical trials.
Inclusion is better than the absence of data on their treatment, said Catherine Spong, former chair of the federal task force.
"It's a paradox," said Spong, professor of obstetrics and gynecology and chief of maternal fetal medicine at University of Texas Southwestern Medical Center. "There is a desire to protect women and fetuses from harm, which is translated to a reluctance to include them in research. By excluding them, the evidence for their care is limited."
Jacqueline Wolf, a professor of the history of medicine at Ohio University, agreed.
"In excluding pregnant women from drug trials to protect them from experimentation, we subject them to uncontrolled experimentation," she said. "We give them the medication without doing any research, and that's dangerous."
Women, of course, don't stop getting sick or having chronic medical conditions just because they are pregnant or breastfeeding, and conditions during pregnancy can affect a baby's health later in life. Evidence-based data is important for other reasons, too.
Pregnancy can dramatically change a woman's physiology, affecting how drugs act on her body and how her body acts or reacts to drugs. For instance, pregnant bodies can more quickly clear out medications such as glyburide, used during diabetes in pregnancy to stabilize high blood-sugar levels, which can be toxic to the fetus and harmful to women. That means a regular dose of the drug may not be enough to control blood sugar and prevent poor outcomes.
Pregnant patients also may be reluctant to take needed drugs for underlying conditions (and doctors may be hesitant to prescribe them), which in turn can cause more harm to the woman and fetus than had they been treated. For example, women who have severe asthma attacks while pregnant are at a higher risk of having low-birthweight babies, and pregnant women with uncontrolled diabetes in early pregnancy have more than four times the risk of birth defects.
Current clinical trials involving pregnant women are assessing treatments for obstructive sleep apnea, postpartum hemorrhage, lupus, and diabetes.
For Kate O'Brien, taking medication during her pregnancy was a matter of life and death. A freelance video producer who lives in New Jersey, O'Brien was diagnosed with tuberculosis in 2015 after she became pregnant with her second child, a boy. Even as she signed hospital consent forms, she had no idea if the treatment would harm him.
"It's a really awful experience," said O'Brien, who now is active with We are TB, an advocacy and support network. "All they had to tell me about the medication was just that women have been taking it for a really long time all over the world. That was the best they could do."
More and more doctors, researchers and women's health organizations and advocates are calling that unacceptable.
By indicating that filling current knowledge gaps is "a critical public health need," the FDA is signaling its support for advancing research with pregnant women, said Lyerly, also co-founder of the Second Wave Initiative, which promotes fair representation of the health interests of pregnant women in biomedical research and policies. "It's a very important shift."
Research with pregnant women can be done ethically, Lyerly said, whether by systematically collecting data from those already taking medications or enrolling pregnant women in studies of drugs or vaccines in development.
Current clinical trials involving pregnant women are assessing treatments for obstructive sleep apnea, postpartum hemorrhage, lupus, and diabetes. Notable trials in development target malaria and HIV prevention in pregnancy.
"It clearly is doable to do this research, and test trials are important to provide evidence for treatment," Spong said. "If we don't have that evidence, we aren't making the best educated decisions for women."

