The Top 8 Things to Know About Anti-Aging Research Right Now
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

A birthday celebration in the future for someone reaching 150 years old.
Dr. Michael West has a storied legacy in the world of aging research. Twenty years ago, the company he started, Geron, hit upon a major breakthrough when his scientists isolated the active component for the gene that confers immortality to cells, called telomerase.
In the twenty years since, a new field has emerged: the science of extending the human "healthspan."
He was in the lab when scientists for the first time artificially turned on the gene in some skin cells donated by Dr. Leonard Hayflick, the man who had discovered back in 1965 that human cells age over time. Sure enough, with Geron's intervention, Hayflick's skin cells became immortal in the dish, and the landmark paper was published in Science in 1998.
In the twenty years since, a new field has emerged: the science of extending the human "healthspan" – the length of time people can live free of diseases related to aging. A substantial amount of preclinical and some clinical research is now underway, backed by heavy investments from some of the world's largest companies.
Today, Dr. West is the CEO of AgeX Therapeutics, a biotech company that is developing novel therapeutics to target human aging and age-related degenerative diseases using pluripotent stem cells. Dr. West recently shared some key insights with Editor-in-Chief Kira Peikoff about what's happening in this exciting space.
1) Pluripotent stem cells have opened the door for the first time in human history to manufacturing young cells and young tissue of any kind.
These are the body's master cells: They are self-replicating, and they can potentially give rise to any cell or tissue the body needs to repair itself. This year marks the 20th anniversary since their isolation for the first time in a lab.
"People in biotech say that the time from lab to discovery in products is about 20 years," West says. "But the good news is we're at that 20-year mark now, so you're seeing an explosive growth of applications. We can now make all cell types of the human body in a scalable manner."
2) Early human development could hold the key to unlocking the mystery of aging.
West believes that two things occur when the body forms in utero: telomerase, the immortalizing gene, gets turned off very early in development in the body cells like skin, liver, and nerves. Additionally, he thinks that a second genetic switch gets turned off that holds the potential for regeneration after injury.
"These insights open the door to intervention by the transfer of telomerase into the cells of the body."
"Very early when the body is first forming, if you cut the skin, it will not respond by scarring, but will regenerate scarlessly," he says. "But that potential gets turned off once the body is formed, about 8 weeks after fertilization. Then, you accumulate damage over a lifetime. Not only do cells have a finite capacity to replicate, but you have tissue damage."
However, there are animals in nature whose telomerase is never turned off, or whose regenerative ability is never turned off. The flatworm, for example, can regenerate its own head if it gets cut off, and it also shows no detectable aging. Lobsters are believed to be similar. (That's not to say it can't get caught and eaten for dinner.)
"These insights open the door to intervention by the transfer of telomerase into the cells of the body, or understanding how regeneration gets turned off, and then turning it back on," West says. "That's well within the power of modern medical research to understand."
3) Companies are investing tremendous resources into the anti-aging gold rush.
Devising interventions is the mission of AgeX, a subsidiary of BioTime, as well as a number of other companies.
"We're seeing a mad rush," West says. There's Google's Calico, which recently announced, with AbbVie Inc., another $1 billion into research for age-related diseases, on top of the previous $1.5 billion investment.
Other notable players include Unity Biotechnology, Samumed, Human Longevity Inc., RestorBio, Rejuvenate Bio,and Juvenescence (which is also an investor in AgeX).
"These are products in development by our company and others that the baby boomers can reasonably anticipate being available within their lifetimes."
4) The majority of clinical applications are still years away.
"What we've learned about turning back on this regenerative state, called induced tissue regeneration, is that the majority of the clinical implications are years away and will require years of clinical trials before potential FDA approval and marketing to the public," West says. "But we have found some potential near-term applications that we think may have a much faster track to commercialization. As you can imagine, we are all over those."
BioTime, Inc., AgeX's parent, has a regenerative medicine product in clinical trials for age-related macular degeneration, the leading cause of blindness in an aging population. While not yet approved by the FDA, BioTime has reported continued progress in the clinical development of the product now in Phase II trials.
Citi recently issued a major report, Disruptive Innovations VI, that included "Anti-Aging Medicines" as the number two innovation for investors to keep an eye on, and predicted that the first anti-aging therapies could receive regulatory approval by 2023.
5) Few, if any, medical interventions are available today that are proven to markedly slow aging - yet. But the Baby Boomers are not necessarily out of luck.
Buyer beware of any claims in the marketplace that a given skin cream or stem cell product will extend your life. More than likely, they won't.
"There are a lot of people trying to cash in on the aging baby boom population," West warns.
"When you hear claims of stem cell products that you can get now, it's important to understand that they are likely not based on pluripotent stem cell technology. Also, they are usually not products approved by the FDA, having gone through clinical trials to demonstrate safety and efficacy."
However, an array of young pluripotent stem cell-derived therapies are on a development track for future approvals.
One example is another program at AgeX: the manufacture of brown fat cells; these cells burn calories rather than store them. They burn circulating fat like triglycerides and sugar in the blood and generate heat.
"You lose brown fat in aging, and animal models suggest that if you restore that tissue, you can restore a metabolic balance to be more like what you had when you were young," says West. "When I was 18, I could drink milkshakes all day long and not gain an ounce. But at 50 or 60, most of us would rapidly put on weight. Why? We believe that one important factor is that with age, you lose this brown fat tissue. The loss throws your metabolism off balance. So the solution is conceptually simple, we plan to make young brown fat cells for transplantation to reset the balance, potentially to treat Type II diabetes or even obesity.
"These are products in development by our company and others that the baby boomers can reasonably anticipate being available within their lifetimes."
6) There is an ethical debate about how far to apply this new science.
Some people are speculating about whether genetic engineering might one day be used to program longer lifespans into humans at the earliest stages of development. (Note: it is against the law across the Western world to edit human embryos intended for reproduction, although just last week, Chinese scientists used CRISPR to repair a disease-causing mutation in viable human embryos.)
West sounds a cautionary note about such interventions meant to lengthen life. "For people who think not just about the science, but the ethics, safety is a major concern. It's entirely possible to genetically engineer babies, but when you make such modifications, it's an experiment, not just in human cells in a dish, but in a human being. I have a great reticence to put any human at risk unless it's a case where the person is suffering with a life-threatening disease, and the potential therapy is their last best hope."
"I have no doubt, zero doubt, that in the foreseeable future, we'll hear of a person who has lived to about 150."
7) The biggest challenge of intervening in human aging is cultural denial.
"The prospect of intervening in a profound way in human aging is still not seen as credible by the vast majority of thoughtful people around the world," West laments.
"Aging is a universal phenomenon, it's mankind's greatest enemy, but as a species we've adapted to the realities of finite lifespans and death. We have a whole infrastructure of belief systems around this, and many people see it as inevitable."
8) The lifespan for healthy children born today could surpass anything humanity has ever seen.
"It is at least 150 years of age," West predicts. "I have no doubt, zero doubt, that in the foreseeable future, we'll hear of a person who has lived to about 150. We know now it's possible. I've never said that publicly before, but I am comfortable now with the prediction. And, of course, if some people now living could live to 150 years of age, we have the prospect of them living to see even more powerful therapies. So, the question now is, what kind of a world are we going to make for future generations?"
[Editor's Note: Check out our latest video, which was inspired by Dr. West's exclusive prediction to leapsmag.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
DNA- and RNA-based electronic implants may revolutionize healthcare
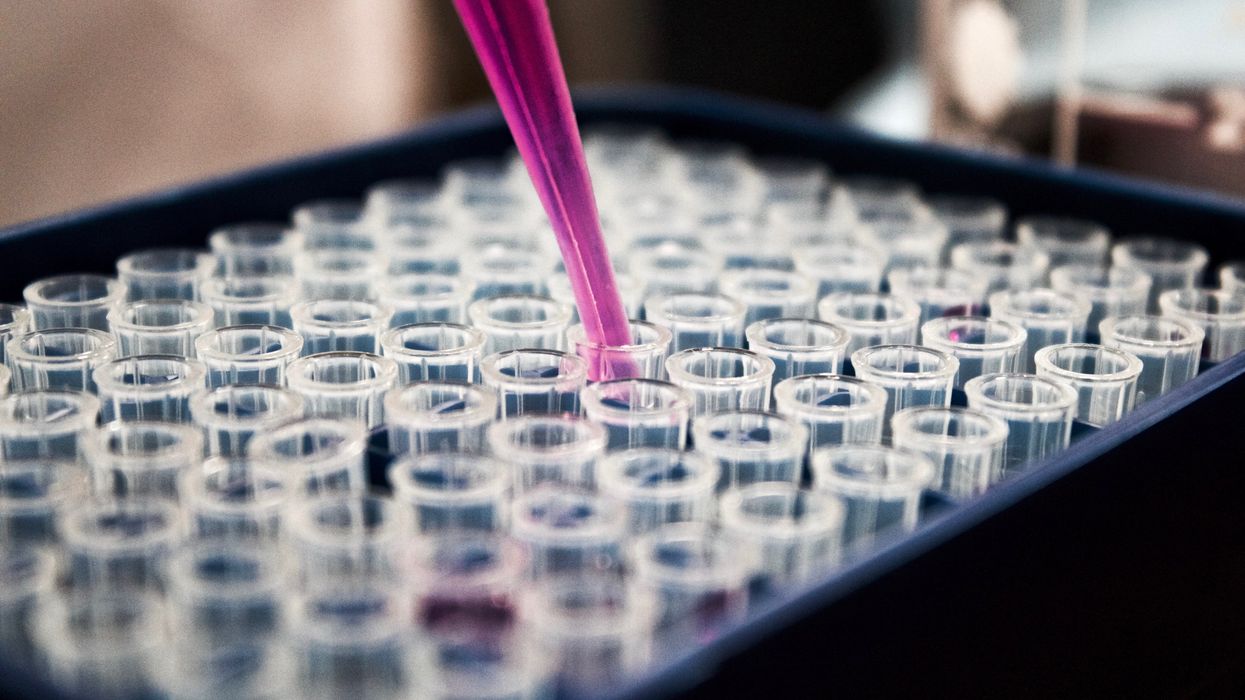
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
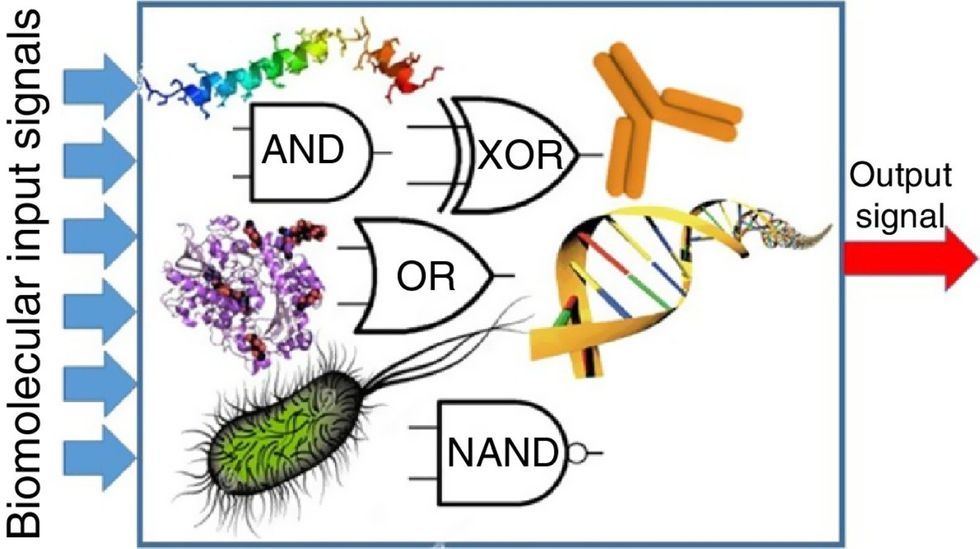
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.