These Sisters May Change the Way You Think About Dying

Anita Freeman, left, hugs her sister Elizabeth Martin at a bakery in Huntington Beach, California, in 2009 — a year before Elizabeth's cancer diagnosis. (Courtesy Anita Freeman)
For five weeks, Anita Freeman watched her sister writhe in pain. The colon cancer diagnosed four years earlier became metastatic.
"I still wouldn't wish that ending on my worst enemy."
At this tormenting juncture, her 66-year-old sister, Elizabeth Martin, wanted to die comfortably in her sleep. But doctors wouldn't help fulfill that final wish.
"It haunts me," Freeman, 74, who lives in Long Beach, California, says in recalling the prolonged agony. Her sister "was breaking out of the house and running in her pajamas down the sidewalk, screaming, 'Help me. Help me.' She just went into a total panic."
Finally, a post-acute care center offered pentobarbital, a sedative that induced a state of unconsciousness, but only after an empathetic palliative care doctor called and insisted on ending the inhumane suffering. "We even had to fight the owners of the facility to get them to agree to the recommendations," Freeman says, describing it as "the only option we had at that time; I still wouldn't wish that ending on my worst enemy."
Her sister died a week later, in 2014. That was two years before California's medical aid-in-dying law took effect, making doctors less reliant on palliative sedation to peacefully end unbearable suffering for terminally ill patients. Now, Freeman volunteers for Compassion & Choices, a national grassroots organization based in Portland, Oregon, that advocates for expanding end-of-life options.
Palliative sedation involves medicating a terminally ill patient into lowered awareness or unconsciousness in order to relieve otherwise intractable suffering at the end of life. It is not intended to cause death, which occurs due to the patient's underlying disease.
In contrast, euthanasia involves directly and deliberately ending a patient's life. Euthanasia is legal only in Canada and some European countries and requires a health care professional to administer the medication. In the United States, laws in seven states and Washington, D.C. give terminally ill patients the option to obtain prescription medication they can take to die peacefully in their sleep, but they must be able to self-adminster it.
Recently, palliative sedation has been gaining more acceptance among medical professionals as an occasional means to relieve suffering, even if it may advance the time of death, as some clinicians believe. However, studies have found no evidence of this claim. Many doctors and bioethicists emphasize that intent is what distinguishes palliative sedation from euthanasia. Others disagree. It's common for controversy to swirl around when and how to apply this practice.

Elizabeth Martin with her sister Anita Freeman in happier times, before metastatic cancer caused her tremendous suffering at the end of her life.
(Courtesy Anita Freeman)
"Intent is everything in ethics. The rigor and protocols we have around palliative sedation therapy also speaks to it being an intervention directed to ease refractory distress," says Martha Twaddle, medical director of palliative medicine and supportive care at Northwestern University's Lake Forest Hospital in Lake Forest, Illinois.
Palliative sedation should be considered only when pain, shortness of breath, and other unbearable symptoms don't respond to conventional treatments. Left to his or her own devices, a patient in this predicament could become restless, Twaddle says, noting that "agitated delirium is a horrible symptom for a family to witness."
At other times, "we don't want to be too quick to sedate," particularly in cases of purely "existential distress"—when a patient experiences anticipatory grief around "saying goodbye" to loved ones, she explains. "We want to be sure we're applying the right therapy for the problem."
Encouraging patients to reconcile with their kin may help them find inner peace. Nonmedical interventions worth exploring include quieting the environment and adjusting lighting to simulate day and night, Twaddle says.
Music-thanatology also can have a calming effect. It is live, prescriptive music, mainly employing the harp or voice, tailored to the patient's physiological needs by tuning into vital signs such as heart rate, respiration, and temperature, according to the Music-Thanatology Association International.
"When we integrated this therapeutic modality in 2003, our need for using palliative sedation therapy dropped 75 percent and has remained low ever since," Twaddle observes. "We have this as part of our care for treating refractory symptoms."
"If palliative sedation is being employed properly with the right patient, it should not hasten death."
Ethical concerns surrounding euthanasia often revolve around the term "terminal sedation," which "can entail a physician deciding that the patient is a lost cause—incurable medically and in substantial pain that cannot adequately be relieved," says John Kilner, professor and director of the bioethics programs at Trinity International University in Deerfield, Illinois.
By halting sedation at reasonable intervals, the care team can determine whether significant untreatable pain persists. Periodic discontinuation serves as "evidence that the physician is still working to restore the patient rather than merely to usher the patient painlessly into death," Kilner explains. "Indeed, sometimes after a period of unconsciousness, with the body relieved of unceasing pain, the body can recover enough to make the pain treatable."
The medications for palliative sedation "are tried and true sedatives that we've had for a long time, for many years, so they're predictable," says Joe Rotella, chief medical officer at the American Academy of Hospice and Palliative Medicine.
Some patients prefer to keep their eyes open and remain conscious to answer by name, while others tell their doctors in advance that they want to be more heavily sedated while receiving medications to manage pain and other symptoms. "We adjust the dosage until the patient is sleeping at a desired level of sedation," Rotella says.
Sedation is an intrinsic side effect of most medications prescribed to control severe symptoms in terminally ill patients. In general, most people die in a sleepy state, except for instances of sudden, dramatic death resulting from a major heart attack or stroke, says Ryan R. Nash, a palliative medicine physician and director of The Ohio State University Center for Bioethics in Columbus.
"Using those medications to treat pain or shortness of breath is not palliative sedation," Nash says. In addition, providing supplemental nutrition and hydration in situations where death is imminent—with a prognosis limited to hours or days—generally doesn't help prolong life. "If palliative sedation is being employed properly with the right patient," he adds, "it should not hasten death."
Nonetheless, hospice nurses sometimes feel morally distressed over carrying out palliative sedation. Implementing protocols at health systems would help guide them and alleviate some of their concerns, says Gregg VandeKieft, medical director for palliative care at Providence St. Joseph Health's Southwest Washington Region in Olympia, Washington. "It creates guardrails by sort of standardizing and normalizing things," he says.
"Our goal is to restore our patient. It's never to take their life."
The concept of proportionality weighs heavily in the process of palliative sedation. But sometimes substantial doses are necessary. For instance, an opioid-tolerant patient recently needed an unusually large amount of medication to control symptoms. She was in a state of illness-induced confusion and pain, says David E. Smith, a palliative medicine physician at Baptist Health Supportive Care in Little Rock, Arkansas.
Still, "we are parsimonious in what we do. We only use as much therapeutic force as necessary to achieve our goals," Smith says. "Our goal is to restore our patient. It's never to take their life."
Scientists have known about and studied heart rate variability, or HRV, for a long time and, in recent years, monitors have come to market that can measure HRV accurately.
This episode is about a health metric you may not have heard of before: heart rate variability, or HRV. This refers to the small changes in the length of time between each of your heart beats.
Scientists have known about and studied HRV for a long time. In recent years, though, new monitors have come to market that can measure HRV accurately whenever you want.
Five months ago, I got interested in HRV as a more scientific approach to finding the lifestyle changes that work best for me as an individual. It's at the convergence of some important trends in health right now, such as health tech, precision health and the holistic approach in systems biology, which recognizes how interactions among different parts of the body are key to health.
But HRV is just one of many numbers worth paying attention to. For this episode of Making Sense of Science, I spoke with psychologist Dr. Leah Lagos; Dr. Jessilyn Dunn, assistant professor in biomedical engineering at Duke; and Jason Moore, the CEO of Spren and an app called Elite HRV. We talked about what HRV is, research on its benefits, how to measure it, whether it can be used to make improvements in health, and what researchers still need to learn about HRV.
*Talk to your doctor before trying anything discussed in this episode related to HRV and lifestyle changes to raise it.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Show notes
Spren - https://www.spren.com/
Elite HRV - https://elitehrv.com/
Jason Moore's Twitter - https://twitter.com/jasonmooreme?lang=en
Dr. Jessilyn Dunn's Twitter - https://twitter.com/drjessilyn?lang=en
Dr. Dunn's study on HRV, flu and common cold - https://jamanetwork.com/journals/jamanetworkopen/f...
Dr. Leah Lagos - https://drleahlagos.com/
Dr. Lagos on Star Talk - https://www.youtube.com/watch?v=jC2Q10SonV8
Research on HRV and intermittent fasting - https://pubmed.ncbi.nlm.nih.gov/33859841/
Research on HRV and Mediterranean diet - https://medicalxpress.com/news/2010-06-twin-medite...:~:text=Using%20data%20from%20the%20Emory,eating%20a%20Western%2Dtype%20diet
Devices for HRV biofeedback - https://elitehrv.com/heart-variability-monitors-an...
Benefits of HRV biofeedback - https://pubmed.ncbi.nlm.nih.gov/32385728/
HRV and cognitive performance - https://www.frontiersin.org/articles/10.3389/fnins...
HRV and emotional regulation - https://pubmed.ncbi.nlm.nih.gov/36030986/
Fortune article on HRV - https://fortune.com/well/2022/12/26/heart-rate-var...
Peanut allergies affect about a million children in the U.S., and most never outgrow them. Luckily, some promising remedies are in the works.
Ever since he was a baby, Sharon Wong’s son Brandon suffered from rashes, prolonged respiratory issues and vomiting. In 2006, as a young child, he was diagnosed with a severe peanut allergy.
"My son had a history of reacting to traces of peanuts in the air or in food,” says Wong, a food allergy advocate who runs a blog focusing on nut free recipes, cooking techniques and food allergy awareness. “Any participation in school activities, social events, or travel with his peanut allergy required a lot of preparation.”
Peanut allergies affect around a million children in the U.S. Most never outgrow the condition. The problem occurs when the immune system mistakenly views the proteins in peanuts as a threat and releases chemicals to counteract it. This can lead to digestive problems, hives and shortness of breath. For some, like Wong’s son, even exposure to trace amounts of peanuts could be life threatening. They go into anaphylactic shock and need to take a shot of adrenaline as soon as possible.
Typically, people with peanut allergies try to completely avoid them and carry an adrenaline autoinjector like an EpiPen in case of emergencies. This constant vigilance is very stressful, particularly for parents with young children.
“The search for a peanut allergy ‘cure’ has been a vigorous one,” says Claudia Gray, a pediatrician and allergist at Vincent Pallotti Hospital in Cape Town, South Africa. The closest thing to a solution so far, she says, is the process of desensitization, which exposes the patient to gradually increasing doses of peanut allergen to build up a tolerance. The most common type of desensitization is oral immunotherapy, where patients ingest small quantities of peanut powder. It has been effective but there is a risk of anaphylaxis since it involves swallowing the allergen.
"By the end of the trial, my son tolerated approximately 1.5 peanuts," Sharon Wong says.
DBV Technologies, a company based in Montrouge, France has created a skin patch to address this problem. The Viaskin Patch contains a much lower amount of peanut allergen than oral immunotherapy and delivers it through the skin to slowly increase tolerance. This decreases the risk of anaphylaxis.
Wong heard about the peanut patch and wanted her son to take part in an early phase 2 trial for 4-to-11-year-olds.
“We felt that participating in DBV’s peanut patch trial would give him the best chance at desensitization or at least increase his tolerance from a speck of peanut to a peanut,” Wong says. “The daily routine was quite simple, remove the old patch and then apply a new one. By the end of the trial, he tolerated approximately 1.5 peanuts.”
How it works
For DBV Technologies, it all began when pediatric gastroenterologist Pierre-Henri Benhamou teamed up with fellow professor of gastroenterology Christopher Dupont and his brother, engineer Bertrand Dupont. Together they created a more effective skin patch to detect when babies have allergies to cow's milk. Then they realized that the patch could actually be used to treat allergies by promoting tolerance. They decided to focus on peanut allergies first as the more dangerous.
The Viaskin patch utilizes the fact that the skin can promote tolerance to external stimuli. The skin is the body’s first defense. Controlling the extent of the immune response is crucial for the skin. So it has defense mechanisms against external stimuli and can promote tolerance.
The patch consists of an adhesive foam ring with a plastic film on top. A small amount of peanut protein is placed in the center. The adhesive ring is attached to the back of the patient's body. The peanut protein sits above the skin but does not directly touch it. As the patient sweats, water droplets on the inside of the film dissolve the peanut protein, which is then absorbed into the skin.
The peanut protein is then captured by skin cells called Langerhans cells. They play an important role in getting the immune system to tolerate certain external stimuli. Langerhans cells take the peanut protein to lymph nodes which activate T regulatory cells. T regulatory cells suppress the allergic response.
A different patch is applied to the skin every day to increase tolerance. It’s both easy to use and convenient.
“The DBV approach uses much smaller amounts than oral immunotherapy and works through the skin significantly reducing the risk of allergic reactions,” says Edwin H. Kim, the division chief of Pediatric Allergy and Immunology at the University of North Carolina, U.S., and one of the principal investigators of Viaskin’s clinical trials. “By not going through the mouth, the patch also avoids the taste and texture issues. Finally, the ability to apply a patch and immediately go about your day may be very attractive to very busy patients and families.”
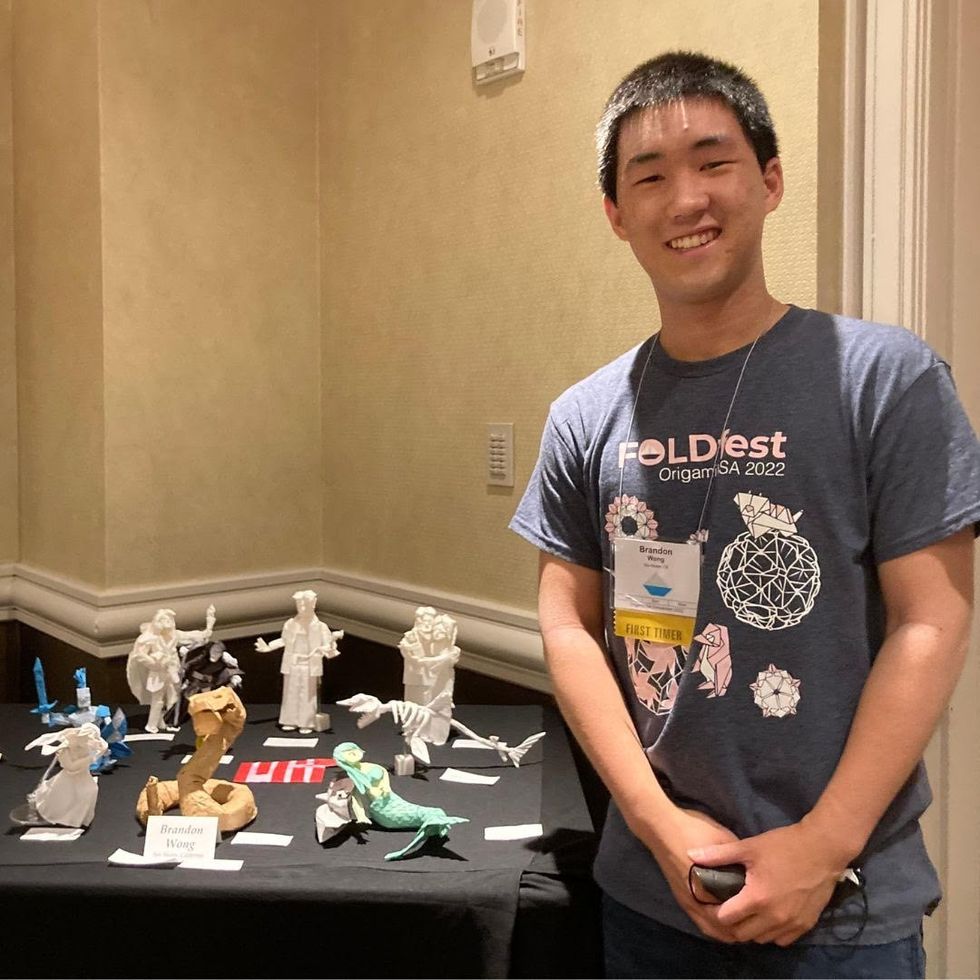
Brandon Wong displaying origami figures he folded at an Origami Convention in 2022
Sharon Wong
Clinical trials
Results from DBV's phase 3 trial in children ages 1 to 3 show its potential. For a positive result, patients who could not tolerate 10 milligrams or less of peanut protein had to be able to manage 300 mg or more after 12 months. Toddlers who could already tolerate more than 10 mg needed to be able to manage 1000 mg or more. In the end, 67 percent of subjects using the Viaskin patch met the target as compared to 33 percent of patients taking the placebo dose.
“The Viaskin peanut patch has been studied in several clinical trials to date with promising results,” says Suzanne M. Barshow, assistant professor of medicine in allergy and asthma research at Stanford University School of Medicine in the U.S. “The data shows that it is safe and well-tolerated. Compared to oral immunotherapy, treatment with the patch results in fewer side effects but appears to be less effective in achieving desensitization.”
The primary reason the patch is less potent is that oral immunotherapy uses a larger amount of the allergen. Additionally, absorption of the peanut protein into the skin could be erratic.
Gray also highlights that there is some tradeoff between risk and efficacy.
“The peanut patch is an exciting advance but not as effective as the oral route,” Gray says. “For those patients who are very sensitive to orally ingested peanut in oral immunotherapy or have an aversion to oral peanut, it has a use. So, essentially, the form of immunotherapy will have to be tailored to each patient.” Having different forms such as the Viaskin patch which is applied to the skin or pills that patients can swallow or dissolve under the tongue is helpful.
The hope is that the patch’s efficacy will increase over time. The team is currently running a follow-up trial, where the same patients continue using the patch.
“It is a very important study to show whether the benefit achieved after 12 months on the patch stays stable or hopefully continues to grow with longer duration,” says Kim, who is an investigator in this follow-up trial.
"My son now attends university in Massachusetts, lives on-campus, and eats dorm food. He has so much more freedom," Wong says.
The team is further ahead in the phase 3 follow-up trial for 4-to-11-year-olds. The initial phase 3 trial was not as successful as the trial for kids between one and three. The patch enabled patients to tolerate more peanuts but there was not a significant enough difference compared to the placebo group to be definitive. The follow-up trial showed greater potency. It suggests that the longer patients are on the patch, the stronger its effects.
They’re also testing if making the patch bigger, changing the shape and extending the minimum time it’s worn can improve its benefits in a trial for a new group of 4-to-11 year-olds.
The future
DBV Technologies is using the skin patch to treat cow’s milk allergies in children ages 1 to 17. They’re currently in phase 2 trials.
As for the peanut allergy trials in toddlers, the hope is to see more efficacy soon.
For Wong’s son who took part in the earlier phase 2 trial for 4-to-11-year-olds, the patch has transformed his life.
“My son continues to maintain his peanut tolerance and is not affected by peanut dust in the air or cross-contact,” Wong says. ”He attends university in Massachusetts, lives on-campus, and eats dorm food. He still carries an EpiPen but has so much more freedom than before his clinical trial. We will always be grateful.”

