This “Absolutely Tireless” Researcher Made an Important Breakthrough for Cancer Patients

Theo Roth.
After months of looking at dead cells under a microscope, Theo Roth finally glimpsed what he had been hoping to see—flickers of green. His method was working.
"If we can go into the cell and add in new code and instructions, now we can give it whatever new functions we want."
When Roth joined the laboratory of Alex Marson at the University of California, San Francisco in June 2016, he set to work trying to figure out a new way to engineer human T cells, a type of white blood cell that's an important part of the immune system. If he succeeded, the resulting approach could make it easier and faster for scientists to develop and test cell and gene therapies, new treatments that involve genetically reprogramming the body's own cells.
For decades, researchers have been using engineered viruses to bestow human cells with new genetic characteristics. These so-called viral vectors "infect" human cells, transferring whatever new genetic material scientists put into them. The idea is that this new DNA could give T cells a boost to better fight diseases like cancer and HIV.
Several successful clinical trials have used virally-modified human T cells, and in fact, the U.S. Food and Drug Administration last year approved two such groundbreaking cancer gene therapies, Kymriah and Yescarta. But the process of genetically manipulating cells with viruses is expensive and time-consuming. In addition, viruses tend to randomly insert DNA with little predictability.
"What Theo wanted to do was to paste in big sequences of DNA at a targeted site without viruses," says Marson, an associate professor of microbiology and immunology. "That would have the benefit of being able to rewrite a specific site in the genome and do it flexibly and quickly without having to make a new virus for every site you want to manipulate."
Scientists have for a while been interested in non-viral engineering methods, but T cells are fragile and notoriously difficult to work with.
Previously, Marson's lab had collaborated with CRISPR pioneer Jennifer Doudna and her team at the University of California, Berkeley to use an electrical pulse together with CRISPR components to knock out certain genes. They also found some success with inserting very small pieces of DNA into a targeted site.
But Roth, a 27-year-old graduate student at UCSF pursuing MD and PhD degrees, was determined to figure out how to paste in much bigger sequences of genetic information. Marson says it was an "ambitious" goal. Scientists had tried before, but found that stuffing large chunks of DNA into T cells would quickly kill them.
"If we can go into the cell and add in new code and instructions, now we can give it whatever new functions we want," Roth says. "If you can add in new DNA sequences at the site that you want, then you have a much greater capacity to generate a cell that's going to be therapeutic or curative for a disease."
"He has already made his mark on the field."
So Roth began experimenting with hundreds of different variables a week, trying to find the right conditions to allow him to engineer T cells without the need for viruses. To know if the technique was working, Roth and his colleagues used a green fluorescent protein that would be expressed in cells that had successfully been modified.
"We went from having a lot of dead cells that didn't have any green to having maybe 1 percent of them being green," Roth says. "At that stage we got really excited."
After nearly a year of testing, he and collaborators found a combination of T cell ratios and DNA quantity mixed with CRISPR and zaps of electricity that seemed to work. These electrical pulses, called electroporation, deliver a jolt to cells that makes their membranes temporarily more permeable, allowing the CRISPR system to slip through. Once inside cells, CRISPR seeks out a specific place in the genome and makes a programmed, precise edit.
Roth and his colleagues used the approach to repair a genetic defect in T cells taken from children with a rare autoimmune disease and also to supercharge T cells so that they'd seek out and selectively kill human cancer cells while leaving healthy cells intact. In mice transplanted with human melanoma tissue, the edited T cells went to straight to the cancerous cells and attacked them. The findings were published in Nature in July.
Marson and Roth think even a relatively small number of modified T cells could be effective at treating some cancers, infections, and autoimmune diseases.
Roth is now working with the Parker Institute for Cancer Immunotherapy in San Francisco to engineer cells to treat a variety of cancers and hopefully commercialize his technique. Fred Ramsdell, vice president at the Parker Institute, says he's impressed by Roth's work. "He has already made his mark on the field."
Right now, there's a huge manufacturing backlog for viruses. If researchers want to start a clinical trial to test a new gene or cell therapy, they often have to wait a year to get the viruses they need.
"I think the biggest immediate impact is that it will lower the cost of a starting an early phase clinical trial."
Ramsdell says what Roth's findings allow researchers to do is engineer T cells quickly and more efficiently, cutting the time it takes to make them from several months to just a few weeks. That will allow researchers to develop and test several potential therapies in the lab at once.
"I think the biggest immediate impact is that it will lower the cost of a starting an early phase clinical trial," Roth says.
This isn't the first time Roth's work has been in the spotlight. As an undergraduate at Stanford University, he made significant contributions to traumatic brain injury research by developing a mouse model for observing the brain's cellular response to a concussion. He started the research, which was also published in Nature, the summer before entering college while he was an intern in Dorian McGavern's lab at the National Institutes of Health.
When Roth entered UCSF as a graduate student, his scientific interests shifted.
"It's definitely a big leap" from concussion research, says McGavern, who still keeps in touch with Roth. But he says he's not surprised about Roth's path. "He's absolutely tireless when it comes to the pursuit of science."
Roth says he's optimistic about the potential for gene and cell therapies to cure patients. "I want to try to figure out what one of the next therapies we should put into patients should be."
After spaceflight record, NASA looks to protect astronauts on even longer trips
NASA astronaut Frank Rubio floats by the International Space Station’s “window to the world.” Yesterday, he returned from the longest single spaceflight by a U.S. astronaut on record - over one year. Exploring deep space will require even longer missions.
At T-minus six seconds, the main engines of the Atlantis Space Shuttle ignited, rattling its capsule “like a skyscraper in an earthquake,” according to astronaut Tom Jones, describing the 1988 launch. As the rocket lifted off and accelerated to three times the force of Earth's gravity, “It felt as if two of my friends were standing on my chest and wouldn’t get off.” But when Atlantis reached orbit, the main engines cut off, and the astronauts were suddenly weightless.
Since 1961, NASA has sent hundreds of astronauts into space while working to making their voyages safer and smoother. Yet, challenges remain. Weightlessness may look amusing when watched from Earth, but it has myriad effects on cognition, movement and other functions. When missions to space stretch to six months or longer, microgravity can impact astronauts’ health and performance, making it more difficult to operate their spacecraft.
Yesterday, NASA astronaut Frank Rubio returned to Earth after over one year, the longest single spaceflight for a U.S. astronaut. But this is just the start; longer and more complex missions into deep space loom ahead, from returning to the moon in 2025 to eventually sending humans to Mars. To ensure that these missions succeed, NASA is increasing efforts to study the biological effects and prevent harm.
The dangers of microgravity are real
A NASA report published in 2016 details a long list of incidents and near-misses caused – at least partly – by space-induced changes in astronauts’ vision and coordination. These issues make it harder to move with precision and to judge distance and velocity.
According to the report, in 1997, a resupply ship collided with the Mir space station, possibly because a crew member bumped into the commander during the final docking maneuver. This mishap caused significant damage to the space station.
Returns to Earth suffered from problems, too. The same report notes that touchdown speeds during the first 100 space shuttle landings were “outside acceptable limits. The fastest landing on record – 224 knots (258 miles) per hour – was linked to the commander’s momentary spatial disorientation.” Earlier, each of the six Apollo crews that landed on the moon had difficulty recognizing moon landmarks and estimating distances. For example, Apollo 15 landed in an unplanned area, ultimately straddling the rim of a five-foot deep crater on the moon, harming one of its engines.
Spaceflight causes unique stresses on astronauts’ brains and central nervous systems. NASA is working to reduce these harmful effects.
NASA
Space messes up your brain
In space, astronauts face the challenges of microgravity, ionizing radiation, social isolation, high workloads, altered circadian rhythms, monotony, confined living quarters and a high-risk environment. Among these issues, microgravity is one of the most consequential in terms of physiological changes. It changes the brain’s structure and its functioning, which can hurt astronauts’ performance.
The brain shifts upwards within the skull, displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes.
That’s partly because of how being in space alters blood flow. On Earth, gravity pulls our blood and other internal fluids toward our feet, but our circulatory valves ensure that the fluids are evenly distributed throughout the body. In space, there’s not enough gravity to pull the fluids down, and they shift up, says Rachael D. Seidler, a physiologist specializing in spaceflight at the University of Florida and principal investigator on many space-related studies. The head swells and legs appear thinner, causing what astronauts call “puffy face chicken legs.”
“The brain changes at the structural and functional level,” says Steven Jillings, equilibrium and aerospace researcher at the University of Antwerp in Belgium. “The brain shifts upwards within the skull,” displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes. Some of the displaced cerebrospinal fluid goes into cavities within the brain, called ventricles, enlarging them. “The remaining fluids pool near the chest and heart,” explains Jillings. After 12 consecutive months in space, one astronaut had a ventricle that was 25 percent larger than before the mission.
Some changes reverse themselves while others persist for a while. An example of a longer-lasting problem is spaceflight-induced neuro-ocular syndrome, which results in near-sightedness and pressure inside the skull. A study of approximately 300 astronauts shows near-sightedness affects about 60 percent of astronauts after long missions on the International Space Station (ISS) and more than 25 percent after spaceflights of only a few weeks.
Another long-term change could be the decreased ability of cerebrospinal fluid to clear waste products from the brain, Seidler says. That’s because compressing the brain also compresses its waste-removing glymphatic pathways, resulting in inflammation, vulnerability to injuries and worsening its overall health.
The effects of long space missions were best demonstrated on astronaut twins Scott and Mark Kelly. This NASA Twins Study showed multiple, perhaps permanent, changes in Scott after his 340-day mission aboard the ISS, compared to Mark, who remained on Earth. The differences included declines in Scott’s speed, accuracy and cognitive abilities that persisted longer than six months after returning to Earth in March 2016.
By the end of 2020, Scott’s cognitive abilities improved, but structural and physiological changes to his eyes still remained, he said in a BBC interview.
“It seems clear that the upward shift of the brain and compression of the surrounding tissues with ventricular expansion might not be a good thing,” Seidler says. “But, at this point, the long-term consequences to brain health and human performance are not really known.”

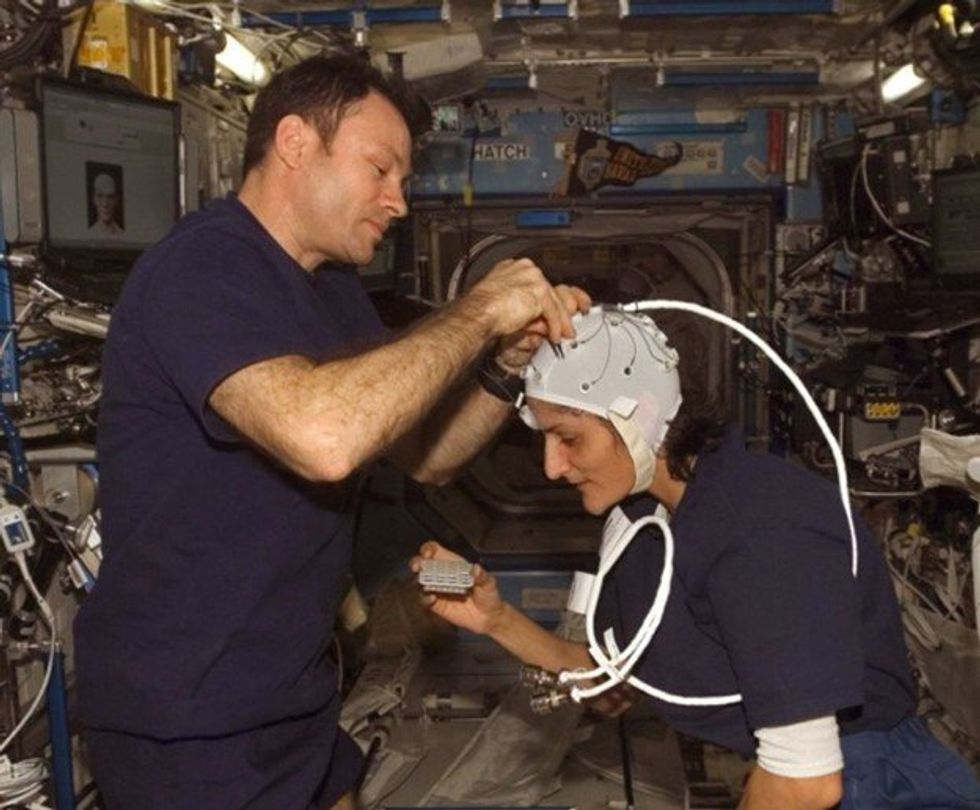
NASA astronaut Kate Rubins conducts a session for the Neuromapping investigation.
NASA
Staying sharp in space
To investigate how prolonged space travel affects the brain, NASA launched a new initiative called the Complement of Integrated Protocols for Human Exploration Research (CIPHER). “CIPHER investigates how long-duration spaceflight affects both brain structure and function,” says neurobehavioral scientist Mathias Basner at the University of Pennsylvania, a principal investigator for several NASA studies. “Through it, we can find out how the brain adapts to the spaceflight environment and how certain brain regions (behave) differently after – relative to before – the mission.”
To do this, he says, “Astronauts will perform NASA’s cognition test battery before, during and after six- to 12-month missions, and will also perform the same test battery in an MRI scanner before and after the mission. We have to make sure we better understand the functional consequences of spaceflight on the human brain before we can send humans safely to the moon and, especially, to Mars.”
As we go deeper into space, astronauts cognitive and physical functions will be even more important. “A trip to Mars will take about one year…and will introduce long communication delays,” Seidler says. “If you are on that mission and have a problem, it may take eight to 10 minutes for your message to reach mission control, and another eight to 10 minutes for the response to get back to you.” In an emergency situation, that may be too late for the response to matter.
“On a mission to Mars, astronauts will be exposed to stressors for unprecedented amounts of time,” Basner says. To counter them, NASA is considering the continuous use of artificial gravity during the journey, and Seidler is studying whether artificial gravity can reduce the harmful effects of microgravity. Some scientists are looking at precision brain stimulation as a way to improve memory and reduce anxiety due to prolonged exposure to radiation in space.
Other scientists are exploring how to protect neural stem cells (which create brain cells) from radiation damage, developing drugs to repair damaged brain cells and protect cells from radiation.
To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
Additionally, NASA is scrutinizing each aspect of the mission, including astronaut exercise, nutrition and intellectual engagement. “We need to give astronauts meaningful work. We need to stimulate their sensory, cognitive and other systems appropriately,” Basner says, especially given their extreme confinement and isolation. The scientific experiments performed on the ISS – like studying how microgravity affects the ability of tissue to regenerate is a good example.
“We need to keep them engaged socially, too,” he continues. The ISS crew, for example, regularly broadcasts from space and answers prerecorded questions from students on Earth, and can engage with social media in real time. And, despite tight quarters, NASA is ensuring the crew capsule and living quarters on the moon or Mars include private space, which is critical for good mental health.
Exploring deep space builds on a foundation that began when astronauts first left the planet. With each mission, scientists learn more about spaceflight effects on astronauts’ bodies. NASA will be using these lessons to succeed with its plans to build science stations on the moon and, eventually, Mars.
“Through internally and externally led research, investigations implemented in space and in spaceflight simulations on Earth, we are striving to reduce the likelihood and potential impacts of neurostructural changes in future, extended spaceflight,” summarizes NASA scientist Alexandra Whitmire. To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
A newly discovered brain cell may lead to better treatments for cognitive disorders
Swiss researchers have found a type of brain cell that appears to be a hybrid of the two other main types — and it could lead to new treatments for brain disorders.
Swiss researchers have discovered a third type of brain cell that appears to be a hybrid of the two other primary types — and it could lead to new treatments for many brain disorders.
The challenge: Most of the cells in the brain are either neurons or glial cells. While neurons use electrical and chemical signals to send messages to one another across small gaps called synapses, glial cells exist to support and protect neurons.
Astrocytes are a type of glial cell found near synapses. This close proximity to the place where brain signals are sent and received has led researchers to suspect that astrocytes might play an active role in the transmission of information inside the brain — a.k.a. “neurotransmission” — but no one has been able to prove the theory.
A new brain cell: Researchers at the Wyss Center for Bio and Neuroengineering and the University of Lausanne believe they’ve definitively proven that some astrocytes do actively participate in neurotransmission, making them a sort of hybrid of neurons and glial cells.
According to the researchers, this third type of brain cell, which they call a “glutamatergic astrocyte,” could offer a way to treat Alzheimer’s, Parkinson’s, and other disorders of the nervous system.
“Its discovery opens up immense research prospects,” said study co-director Andrea Volterra.
The study: Neurotransmission starts with a neuron releasing a chemical called a neurotransmitter, so the first thing the researchers did in their study was look at whether astrocytes can release the main neurotransmitter used by neurons: glutamate.
By analyzing astrocytes taken from the brains of mice, they discovered that certain astrocytes in the brain’s hippocampus did include the “molecular machinery” needed to excrete glutamate. They found evidence of the same machinery when they looked at datasets of human glial cells.
Finally, to demonstrate that these hybrid cells are actually playing a role in brain signaling, the researchers suppressed their ability to secrete glutamate in the brains of mice. This caused the rodents to experience memory problems.
“Our next studies will explore the potential protective role of this type of cell against memory impairment in Alzheimer’s disease, as well as its role in other regions and pathologies than those explored here,” said Andrea Volterra, University of Lausanne.
But why? The researchers aren’t sure why the brain needs glutamatergic astrocytes when it already has neurons, but Volterra suspects the hybrid brain cells may help with the distribution of signals — a single astrocyte can be in contact with thousands of synapses.
“Often, we have neuronal information that needs to spread to larger ensembles, and neurons are not very good for the coordination of this,” researcher Ludovic Telley told New Scientist.
Looking ahead: More research is needed to see how the new brain cell functions in people, but the discovery that it plays a role in memory in mice suggests it might be a worthwhile target for Alzheimer’s disease treatments.
The researchers also found evidence during their study that the cell might play a role in brain circuits linked to seizures and voluntary movements, meaning it’s also a new lead in the hunt for better epilepsy and Parkinson’s treatments.
“Our next studies will explore the potential protective role of this type of cell against memory impairment in Alzheimer’s disease, as well as its role in other regions and pathologies than those explored here,” said Volterra.