Three Big Biotech Ideas to Watch in 2020—And Beyond

Body-on-a-chip, prime editing, and gut microbes all are poised to make a big impact in 2020.
1. Happening Now: Body-on-a-Chip Technology Is Enabling Safer Drug Trials and Better Cancer Research
Researchers have increasingly used the technology known as "lab-on-a-chip" or "organ-on-a-chip" to test the effects of pharmaceuticals, toxins, and chemicals on humans. Rather than testing on animals, which raises ethical concerns and can sometimes be inaccurate, and human-based clinical trials, which can be expensive and difficult to iterate, scientists turn to tiny, micro-engineered chips—about the size of a thumb drive.
It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip.
The chips are lined with living samples of human cells, which mimic the physiology and mechanical forces experienced by cells inside the human body, down to blood flow and breathing motions; the functions of organs ranging from kidneys and lungs to skin, eyes, and the blood-brain barrier.
A more recent—and potentially even more useful—development takes organ-on-a-chip technology to the next level by integrating several chips into a "body-on-a-chip." Since human organs don't work in isolation, seeing how they all react—and interact—once a foreign element has been introduced can be crucial to understanding how a certain treatment will or won't perform. Dr. Shyni Varghese, a MEDx investigator at the Duke University School of Medicine, is one of the researchers working with these systems in order to gain a more nuanced understanding of how multiple different organs react to the same stimuli.
Her lab is working on "tumor-on-a-chip" models, which can not only show the progression and treatment of cancer, but also model how other organs would react to immunotherapy and other drugs. "The effect of drugs on different organs can be tested to identify potential side effects," Varghese says. In addition, these models can help the researchers figure out how cancers grow and spread, as well as how to effectively encourage immune cells to move in and attack a tumor.
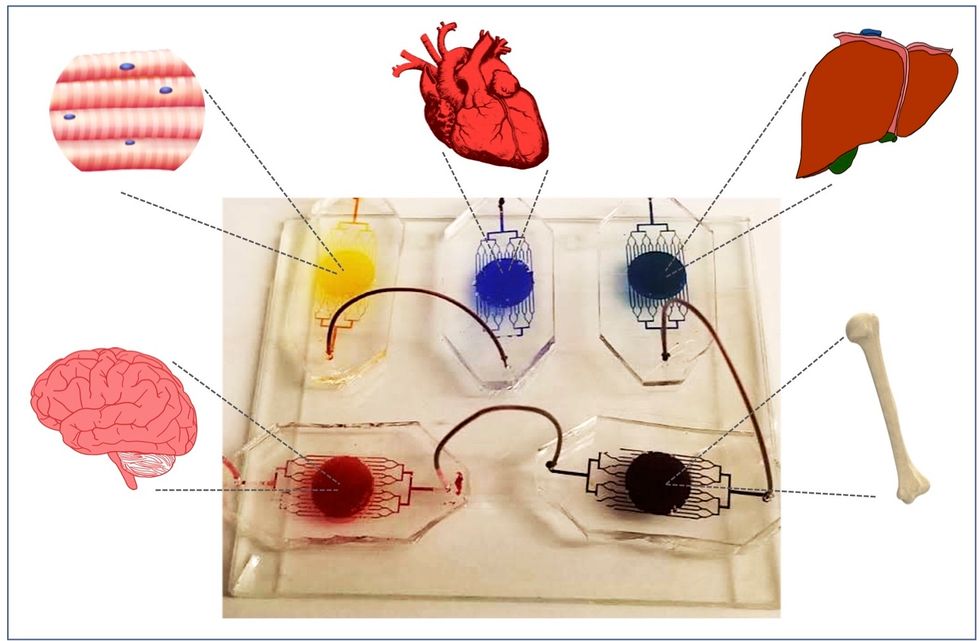
One body-on-a-chip used by Dr. Varghese's lab tracks the interactions of five organs—brain, heart, liver, muscle, and bone.
As their research progresses, Varghese and her team are looking for ways to maintain the long-term function of the engineered organs. In addition, she notes that this kind of research is not just useful for generalized testing; "organ-on-chip technologies allow patient-specific analyses, which can be used towards a fundamental understanding of disease progression," Varghese says. It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip for safety, efficacy, and potential side effects before writing a prescription.
2. Happening Soon: Prime Editing Will Have the Power to "Find and Replace" Disease-Causing Genes
Biochemist David Liu made industry-wide news last fall when he and his lab at MIT's Broad Institute, led by Andrew Anzalone, published a paper on prime editing: a new, more focused technology for editing genes. Prime editing is a descendant of the CRISPR-Cas9 system that researchers have been working with for years, and a cousin to Liu's previous innovation—base editing, which can make a limited number of changes to a single DNA letter at a time.
By contrast, prime editing has the potential to make much larger insertions and deletions; it also doesn't require the tweaked cells to divide in order to write the changes into the DNA, which could make it especially suitable for central nervous system diseases, like Parkinson's.
Crucially, the prime editing technique has a much higher efficiency rate than the older CRISPR system, and a much lower incidence of accidental insertions or deletions, which can make dangerous changes for a patient.
It also has a very broad potential range: according to Liu, 89% of the pathogenic mutations that have been collected in ClinVar (a public archive of human variations) could, in principle, be treated with prime editing—although he is careful to note that correcting a single genetic mutation may not be sufficient to fully treat a genetic disease.
Figuring out just how prime editing can be used most effectively and safely will be a long process, but it's already underway. The same day that Liu and his team posted their paper, they also made the basic prime editing constructs available for researchers around the world through Addgene, a plasmid repository, so that others in the scientific community can test out the technique for themselves. It might be years before human patients will see the results, and in the meantime, significant bioethical questions remain about the limits and sociological effects of such a powerful gene-editing tool. But in the long fight against genetic diseases, it's a huge step forward.
3. Happening When We Fund It: Focusing on Microbiome Health Could Help Us Tackle Social Inequality—And Vice Versa
The past decade has seen a growing awareness of the major role that the microbiome, the microbes present in our digestive tract, play in human health. Having a less-healthy microbiome is correlated with health risks like diabetes and depression, and interventions that target gut health, ranging from kombucha to fecal transplants, have cropped up with increasing frequency.
New research from the University of Maine's Dr. Suzanne Ishaq takes an even broader view, arguing that low-income and disadvantaged populations are less likely to have healthy, diverse gut bacteria, and that increasing access to beneficial microorganisms is an important juncture of social justice and public health.
"Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
"Typically, having a more diverse bacterial community is associated with health, and having fewer different species is associated with illness and may leave you open to infection from bacteria that are good at exploiting opportunities," Ishaq says.
Having a healthy biome doesn't mean meeting one fixed ratio of gut bacteria, since different combinations of microbes can generate roughly similar results when they work in concert. Generally, "good" microbes are the ones that break down fiber and create the byproducts that we use for energy, or ones like lactic acid bacteria that work to make microbials and keep other bacteria in check. The microbial universe in your gut is chaotic, Ishaq says. "Microbes in your gut interact with each other, with you, with your food, or maybe they don't interact at all and pass right through you." Overall, it's tricky to name specific microbial communities that will make or break someone's health.
There are important corollaries between environment and biome health, though, which Ishaq points out: Living in urban environments reduces microbial exposure, and losing the microorganisms that humans typically source from soil and plants can reduce our adaptive immunity and ability to fight off conditions like allergies and asthma. Access to green space within cities can counteract those effects, but in the U.S. that access varies along income, education, and racial lines. Likewise, lower-income communities are more likely to live in food deserts or areas where the cheapest, most convenient food options are monotonous and low in fiber, further reducing microbial diversity.
Ishaq also suggests other areas that would benefit from further study, like the correlation between paid family leave, breastfeeding, and gut microbiota. There are technical and ethical challenges to direct experimentation with human populations—but that's not what Ishaq sees as the main impediment to future research.
"The biggest roadblock is money, and the solution is also money," she says. "Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
That means investment in things we already understand to improve public health, like better education and healthcare, green space, and nutritious food. It also means funding ambitious, interdisciplinary research that will investigate the connections between urban infrastructure, housing policy, social equity, and the millions of microbes keeping us company day in and day out.
Stronger psychedelics that rewire the brain, with Doug Drysdale
Today's podcast episode features Doug Drysdale, CEO of Cybin, a company that is leading innovations in psilocybin, mushrooms that may help people with anxiety and depression.
A promising development in science in recent years has been the use technology to optimize something natural. One-upping nature's wisdom isn't easy. In many cases, we haven't - and maybe we can't - figure it out. But today's episode features a fascinating example: using tech to optimize psychedelic mushrooms.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
These mushrooms have been used for religious, spiritual and medicinal purposes for thousands of years, but only in the past several decades have scientists brought psychedelics into the lab to enhance them and maximize their therapeutic value.
Today’s podcast guest, Doug Drysdale, is doing important work to lead this effort. Drysdale is the CEO of a company called Cybin that has figured out how to make psilocybin more potent, so it can be administered in smaller doses without side effects.
The natural form of psilocybin has been studied increasingly in the realm of mental health. Taking doses of these mushrooms appears to help people with anxiety and depression by spurring the development of connections in the brain, an example of neuroplasticity. The process basically shifts the adult brain from being fairly rigid like dried clay into a malleable substance like warm wax - the state of change that's constantly underway in the developing brains of children.
Neuroplasticity in adults seems to unlock some of our default ways of of thinking, the habitual thought patterns that’ve been associated with various mental health problems. Some promising research suggests that psilocybin causes a reset of sorts. It makes way for new, healthier thought patterns.
So what is Drysdale’s secret weapon to bring even more therapeutic value to psilocybin? It’s a process called deuteration. It focuses on the hydrogen atoms in psilocybin. These atoms are very light and don’t stick very well to carbon, which is another atom in psilocybin. As a result, our bodies can easily breaks down the bonds between the hydrogen and carbon atoms. For many people, that means psilocybin gets cleared from the body too quickly, before it can have a therapeutic benefit.
In deuteration, scientists do something simple but ingenious: they replace the hydrogen atoms with a molecule called deuterium. It’s twice as heavy as hydrogen and forms tighter bonds with the carbon. Because these pairs are so rock-steady, they slow down the rate at which psilocybin is metabolized, so it has more sustained effects on our brains.
Cybin isn’t Drysdale’s first go around at this - far from it. He has over 30 years of experience in the healthcare sector. During this time he’s raised around $4 billion of both public and private capital, and has been named Ernst and Young Entrepreneur of the Year. Before Cybin, he was the founding CEO of a pharmaceutical company called Alvogen, leading it from inception to around $500 million in revenues, across 35 countries. Drysdale has also been the head of mergers and acquisitions at Actavis Group, leading 15 corporate acquisitions across three continents.
In this episode, Drysdale walks us through the promising research of his current company, Cybin, and the different therapies he’s developing for anxiety and depression based not just on psilocybin but another psychedelic compound found in plants called DMT. He explains how they seem to have such powerful effects on the brain, as well as the potential for psychedelics to eventually support other use cases, including helping us strive toward higher levels of well-being. He goes on to discuss his views on mindfulness and lifestyle factors - such as optimal nutrition - that could help bring out hte best in psychedelics.
Show links:
Doug Drysdale full bio
Doug Drysdale twitter
Cybin website
Cybin development pipeline
Cybin's promising phase 2 research on depression
Johns Hopkins psychedelics research and psilocybin research
Mets owner Steve Cohen invests in psychedelic therapies

Doug Drysdale, CEO of Cybin
How the body's immune resilience affects our health and lifespan
Immune cells battle an infection.
Story by Big Think
It is a mystery why humans manifest vast differences in lifespan, health, and susceptibility to infectious diseases. However, a team of international scientists has revealed that the capacity to resist or recover from infections and inflammation (a trait they call “immune resilience”) is one of the major contributors to these differences.
Immune resilience involves controlling inflammation and preserving or rapidly restoring immune activity at any age, explained Weijing He, a study co-author. He and his colleagues discovered that people with the highest level of immune resilience were more likely to live longer, resist infection and recurrence of skin cancer, and survive COVID and sepsis.
Measuring immune resilience
The researchers measured immune resilience in two ways. The first is based on the relative quantities of two types of immune cells, CD4+ T cells and CD8+ T cells. CD4+ T cells coordinate the immune system’s response to pathogens and are often used to measure immune health (with higher levels typically suggesting a stronger immune system). However, in 2021, the researchers found that a low level of CD8+ T cells (which are responsible for killing damaged or infected cells) is also an important indicator of immune health. In fact, patients with high levels of CD4+ T cells and low levels of CD8+ T cells during SARS-CoV-2 and HIV infection were the least likely to develop severe COVID and AIDS.
Individuals with optimal levels of immune resilience were more likely to live longer.
In the same 2021 study, the researchers identified a second measure of immune resilience that involves two gene expression signatures correlated with an infected person’s risk of death. One of the signatures was linked to a higher risk of death; it includes genes related to inflammation — an essential process for jumpstarting the immune system but one that can cause considerable damage if left unbridled. The other signature was linked to a greater chance of survival; it includes genes related to keeping inflammation in check. These genes help the immune system mount a balanced immune response during infection and taper down the response after the threat is gone. The researchers found that participants who expressed the optimal combination of genes lived longer.
Immune resilience and longevity
The researchers assessed levels of immune resilience in nearly 50,000 participants of different ages and with various types of challenges to their immune systems, including acute infections, chronic diseases, and cancers. Their evaluation demonstrated that individuals with optimal levels of immune resilience were more likely to live longer, resist HIV and influenza infections, resist recurrence of skin cancer after kidney transplant, survive COVID infection, and survive sepsis.
However, a person’s immune resilience fluctuates all the time. Study participants who had optimal immune resilience before common symptomatic viral infections like a cold or the flu experienced a shift in their gene expression to poor immune resilience within 48 hours of symptom onset. As these people recovered from their infection, many gradually returned to the more favorable gene expression levels they had before. However, nearly 30% who once had optimal immune resilience did not fully regain that survival-associated profile by the end of the cold and flu season, even though they had recovered from their illness.
Intriguingly, some people who are 90+ years old still have optimal immune resilience, suggesting that these individuals’ immune systems have an exceptional capacity to control inflammation and rapidly restore proper immune balance.
This could suggest that the recovery phase varies among people and diseases. For example, young female sex workers who had many clients and did not use condoms — and thus were repeatedly exposed to sexually transmitted pathogens — had very low immune resilience. However, most of the sex workers who began reducing their exposure to sexually transmitted pathogens by using condoms and decreasing their number of sex partners experienced an improvement in immune resilience over the next 10 years.
Immune resilience and aging
The researchers found that the proportion of people with optimal immune resilience tended to be highest among the young and lowest among the elderly. The researchers suggest that, as people age, they are exposed to increasingly more health conditions (acute infections, chronic diseases, cancers, etc.) which challenge their immune systems to undergo a “respond-and-recover” cycle. During the response phase, CD8+ T cells and inflammatory gene expression increase, and during the recovery phase, they go back down.
However, over a lifetime of repeated challenges, the immune system is slower to recover, altering a person’s immune resilience. Intriguingly, some people who are 90+ years old still have optimal immune resilience, suggesting that these individuals’ immune systems have an exceptional capacity to control inflammation and rapidly restore proper immune balance despite the many respond-and-recover cycles that their immune systems have faced.
Public health ramifications could be significant. Immune cell and gene expression profile assessments are relatively simple to conduct, and being able to determine a person’s immune resilience can help identify whether someone is at greater risk for developing diseases, how they will respond to treatment, and whether, as well as to what extent, they will recover.