Three Big Biotech Ideas to Watch in 2020—And Beyond

Body-on-a-chip, prime editing, and gut microbes all are poised to make a big impact in 2020.
1. Happening Now: Body-on-a-Chip Technology Is Enabling Safer Drug Trials and Better Cancer Research
Researchers have increasingly used the technology known as "lab-on-a-chip" or "organ-on-a-chip" to test the effects of pharmaceuticals, toxins, and chemicals on humans. Rather than testing on animals, which raises ethical concerns and can sometimes be inaccurate, and human-based clinical trials, which can be expensive and difficult to iterate, scientists turn to tiny, micro-engineered chips—about the size of a thumb drive.
It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip.
The chips are lined with living samples of human cells, which mimic the physiology and mechanical forces experienced by cells inside the human body, down to blood flow and breathing motions; the functions of organs ranging from kidneys and lungs to skin, eyes, and the blood-brain barrier.
A more recent—and potentially even more useful—development takes organ-on-a-chip technology to the next level by integrating several chips into a "body-on-a-chip." Since human organs don't work in isolation, seeing how they all react—and interact—once a foreign element has been introduced can be crucial to understanding how a certain treatment will or won't perform. Dr. Shyni Varghese, a MEDx investigator at the Duke University School of Medicine, is one of the researchers working with these systems in order to gain a more nuanced understanding of how multiple different organs react to the same stimuli.
Her lab is working on "tumor-on-a-chip" models, which can not only show the progression and treatment of cancer, but also model how other organs would react to immunotherapy and other drugs. "The effect of drugs on different organs can be tested to identify potential side effects," Varghese says. In addition, these models can help the researchers figure out how cancers grow and spread, as well as how to effectively encourage immune cells to move in and attack a tumor.
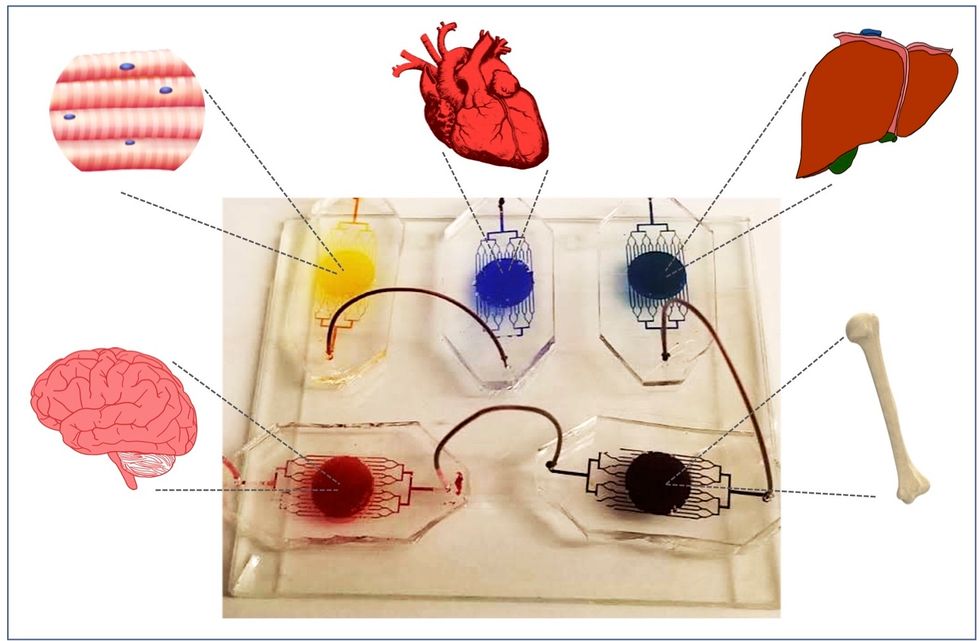
One body-on-a-chip used by Dr. Varghese's lab tracks the interactions of five organs—brain, heart, liver, muscle, and bone.
As their research progresses, Varghese and her team are looking for ways to maintain the long-term function of the engineered organs. In addition, she notes that this kind of research is not just useful for generalized testing; "organ-on-chip technologies allow patient-specific analyses, which can be used towards a fundamental understanding of disease progression," Varghese says. It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip for safety, efficacy, and potential side effects before writing a prescription.
2. Happening Soon: Prime Editing Will Have the Power to "Find and Replace" Disease-Causing Genes
Biochemist David Liu made industry-wide news last fall when he and his lab at MIT's Broad Institute, led by Andrew Anzalone, published a paper on prime editing: a new, more focused technology for editing genes. Prime editing is a descendant of the CRISPR-Cas9 system that researchers have been working with for years, and a cousin to Liu's previous innovation—base editing, which can make a limited number of changes to a single DNA letter at a time.
By contrast, prime editing has the potential to make much larger insertions and deletions; it also doesn't require the tweaked cells to divide in order to write the changes into the DNA, which could make it especially suitable for central nervous system diseases, like Parkinson's.
Crucially, the prime editing technique has a much higher efficiency rate than the older CRISPR system, and a much lower incidence of accidental insertions or deletions, which can make dangerous changes for a patient.
It also has a very broad potential range: according to Liu, 89% of the pathogenic mutations that have been collected in ClinVar (a public archive of human variations) could, in principle, be treated with prime editing—although he is careful to note that correcting a single genetic mutation may not be sufficient to fully treat a genetic disease.
Figuring out just how prime editing can be used most effectively and safely will be a long process, but it's already underway. The same day that Liu and his team posted their paper, they also made the basic prime editing constructs available for researchers around the world through Addgene, a plasmid repository, so that others in the scientific community can test out the technique for themselves. It might be years before human patients will see the results, and in the meantime, significant bioethical questions remain about the limits and sociological effects of such a powerful gene-editing tool. But in the long fight against genetic diseases, it's a huge step forward.
3. Happening When We Fund It: Focusing on Microbiome Health Could Help Us Tackle Social Inequality—And Vice Versa
The past decade has seen a growing awareness of the major role that the microbiome, the microbes present in our digestive tract, play in human health. Having a less-healthy microbiome is correlated with health risks like diabetes and depression, and interventions that target gut health, ranging from kombucha to fecal transplants, have cropped up with increasing frequency.
New research from the University of Maine's Dr. Suzanne Ishaq takes an even broader view, arguing that low-income and disadvantaged populations are less likely to have healthy, diverse gut bacteria, and that increasing access to beneficial microorganisms is an important juncture of social justice and public health.
"Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
"Typically, having a more diverse bacterial community is associated with health, and having fewer different species is associated with illness and may leave you open to infection from bacteria that are good at exploiting opportunities," Ishaq says.
Having a healthy biome doesn't mean meeting one fixed ratio of gut bacteria, since different combinations of microbes can generate roughly similar results when they work in concert. Generally, "good" microbes are the ones that break down fiber and create the byproducts that we use for energy, or ones like lactic acid bacteria that work to make microbials and keep other bacteria in check. The microbial universe in your gut is chaotic, Ishaq says. "Microbes in your gut interact with each other, with you, with your food, or maybe they don't interact at all and pass right through you." Overall, it's tricky to name specific microbial communities that will make or break someone's health.
There are important corollaries between environment and biome health, though, which Ishaq points out: Living in urban environments reduces microbial exposure, and losing the microorganisms that humans typically source from soil and plants can reduce our adaptive immunity and ability to fight off conditions like allergies and asthma. Access to green space within cities can counteract those effects, but in the U.S. that access varies along income, education, and racial lines. Likewise, lower-income communities are more likely to live in food deserts or areas where the cheapest, most convenient food options are monotonous and low in fiber, further reducing microbial diversity.
Ishaq also suggests other areas that would benefit from further study, like the correlation between paid family leave, breastfeeding, and gut microbiota. There are technical and ethical challenges to direct experimentation with human populations—but that's not what Ishaq sees as the main impediment to future research.
"The biggest roadblock is money, and the solution is also money," she says. "Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
That means investment in things we already understand to improve public health, like better education and healthcare, green space, and nutritious food. It also means funding ambitious, interdisciplinary research that will investigate the connections between urban infrastructure, housing policy, social equity, and the millions of microbes keeping us company day in and day out.
A startup aims to make medicines in space
A company is looking to improve medicines by making them in the nearly weightless environment of space.
Story by Big Think
On June 12, a SpaceX Falcon 9 rocket deployed 72 small satellites for customers — including the world’s first space factory.
The challenge: In 2019, pharma giant Merck revealed that an experiment on the International Space Station had shown how to make its blockbuster cancer drug Keytruda more stable. That meant it could now be administered via a shot rather than through an IV infusion.
The key to the discovery was the fact that particles behave differently when freed from the force of gravity — seeing how its drug crystalized in microgravity helped Merck figure out how to tweak its manufacturing process on Earth to produce the more stable version.
Microgravity research could potentially lead to many more discoveries like this one, or even the development of brand-new drugs, but ISS astronauts only have so much time for commercial experiments.
“There are many high-performance products that are only possible to make in zero-gravity, which is a manufacturing capability that cannot be replicated in any factory on Earth.”-- Will Bruey.
The only options for accessing microgravity (or free fall) outside of orbit, meanwhile, are parabolic airplane flights and drop towers, and those are only useful for experiments that require less than a minute in microgravity — Merck’s ISS experiment took 18 days.
The idea: In 2021, California startup Varda Space Industries announced its intention to build the world’s first space factory, to manufacture not only pharmaceuticals but other products that could benefit from being made in microgravity, such as semiconductors and fiber optic cables.
This factory would consist of a commercial satellite platform attached to two Varda-made modules. One module would contain equipment capable of autonomously manufacturing a product. The other would be a reentry capsule to bring the finished goods back to Earth.
“There are many high-performance products that are only possible to make in zero-gravity, which is a manufacturing capability that cannot be replicated in any factory on Earth,” said CEO Will Bruey, who’d previously developed and flown spacecraft for SpaceX.
“We have a team stacked with aerospace talent in the prime of their careers, focused on getting working hardware to orbit as quickly as possible,” he continued.
“[Pharmaceuticals] are the most valuable chemicals per unit mass. And they also have a large market on Earth.” -- Will Bruey, CEO of Varda Space.
What’s new? At the time, Varda said it planned to launch its first space factory in 2023, and, in what feels like a first for a space startup, it has actually hit that ambitious launch schedule.
“We have ACQUISITION OF SIGNAL,” the startup tweeted soon after the Falcon 9 launch on June 12. “The world’s first space factory’s solar panels have found the sun and it’s beginning to de-tumble.”
During the satellite’s first week in space, Varda will focus on testing its systems to make sure everything works as hoped. The second week will be dedicated to heating and cooling the old HIV-AIDS drug ritonavir repeatedly to study how its particles crystalize in microgravity.
After about a month in space, Varda will attempt to bring its first space factory back to Earth, sending it through the atmosphere at hypersonic speeds and then using a parachute system to safely land at the Department of Defense’s Utah Test and Training Range.
Looking ahead: Ultimately, Varda’s space factories could end up serving dual purposes as manufacturing facilities and hypersonic testbeds — the Air Force has already awarded the startup a contract to use its next reentry capsule to test hardware for hypersonic missiles.
But as for manufacturing other types of goods, Varda plans to stick with drugs for now.
“[Pharmaceuticals] are the most valuable chemicals per unit mass,” Bruey told CNN. “And they also have a large market on Earth.”
“You’re not going to see Varda do anything other than pharmaceuticals for the next minimum of six, seven years,” added Delian Asparouhov, Varda’s co-founder and president.
This article originally appeared on Big Think, home of the brightest minds and biggest ideas of all time.

Genes that protect health with Dr. Nir Barzilai
Centenarians essentially won the genetic lottery, says Nir Barzilai of Albert Einstein College of Medicine. He is studying their genes to see how the rest of us can benefit from understanding how they work.
In today’s podcast episode, I talk with Nir Barzilai, a geroscientist, which means he studies the biology of aging. Barzilai directs the Institute for Aging Research at the Albert Einstein College of Medicine.
My first question for Dr. Barzilai was: why do we age? And is there anything to be done about it? His answers were encouraging. We can’t live forever, but we have some control over the process, as he argues in his book, Age Later.
Dr. Barzilai told me that centenarians differ from the rest of us because they have unique gene mutations that help them stay healthy longer. For most of us, the words “gene mutations” spell trouble - we associate these words with cancer or neurodegenerative diseases, but apparently not all mutations are bad.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Centenarians may have essentially won the genetic lottery, but that doesn’t mean the rest of us are predestined to have a specific lifespan and health span, or the amount of time spent living productively and enjoyably. “Aging is a mother of all diseases,” Dr. Barzilai told me. And as a disease, it can be targeted by therapeutics. Dr. Barzilai’s team is already running clinical trials on such therapeutics — and the results are promising.
More about Dr. Barzilai: He is scientific director of AFAR, American Federation for Aging Research. As part of his work, Dr. Barzilai studies families of centenarians and their genetics to learn how the rest of us can learn and benefit from their super-aging. He also organizing a clinical trial to test a specific drug that may slow aging.
Show Links
Age Later: Health Span, Life Span, and the New Science of Longevity https://www.amazon.com/Age-Later-Healthiest-Sharpest-Centenarians/dp/1250230853
American Federation for Aging Research https://www.afar.org
https://www.afar.org/nir-barzilai
https://www.einsteinmed.edu/faculty/484/nir-barzilai/
Metformin as a Tool to Target Aging
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5943638/
Benefits of Metformin in Attenuating the Hallmarks of Aging https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7347426/
The Longevity Genes Project https://www.einsteinmed.edu/centers/aging/longevity-genes-project/
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.