To Save Lives, This Scientist Is Trying to Grow Human Organs Inside of Sheep

A lamb receiving a shot from medical personnel.
More than 114,000 men, women, and children are awaiting organ transplants in the United States. Each day, 22 of them die waiting. To address this shortage, researchers are working hard to grow organs on-demand, using the patient's own cells, to eliminate the need to find a perfectly matched donor.
"The next step is to transplant these cells into a larger animal that will produce an organ that is the right size for a human."
But creating full-size replacement organs in a lab is still decades away. So some scientists are experimenting with the boundaries of nature and life itself: using other mammals to grow human cells. Earlier this year, this line of investigation took a big step forward when scientists announced they had grown sheep embryos that contained human cells.
Dr. Pablo Ross, an associate professor at the University of California, Davis, along with a team of colleagues, introduced human stem cells into the sheep embryos at a very early stage of their development and found that one in every 10,000 cells in the embryo were human. It was an improvement over their prior experiment, using a pig embryo, when they found that one in every 100,000 cells in the pig were human. The resulting chimera, as the embryo is called, is only allowed to develop for 28 days. Leapsmag contributor Caren Chesler recently spoke with Ross about his research. Their interview has been edited and condensed for clarity.
Your goal is to one day grow human organs in animals, for organ transplantation. What does your research entail?
We're transplanting stem cells from a person into an animal embryo, at about day three to five of embryo development.
This concept has already been shown to work between mice and rats. You can grow a mouse pancreas inside a rat, or you can grow a rat pancreas inside a mouse.
For this approach to work for humans, the next step is to transplant these cells into a larger animal that will produce an organ that is the right size for a human. That's why we chose to start some of this preliminary work using pigs and sheep. Adult pigs and adult sheep have organs that are of similar size to an adult human. Pigs and sheep also grow really fast, so they can grow from a single cell at the time of fertilization to human adult size -- about 200 pounds -- in only nine to 10 months. That's better than the average waiting time for an organ transplant.
"You don't want the cells to confer any human characteristics in the animal....Too many cells, that may be a problem, because we do not know what that threshold is."
So how do you get the animal to grow the human organ you want?
First, we need to generate the animal without its own organ. We can generate sheep or pigs that will not grow their own pancreases. Those animals can then be used as hosts for human pancreas generation.
For the approach to work, we need the human stem cells to be able to integrate into the embryo and to contribute to its tissues. What we've been doing with pigs, and more recently, in sheep, is testing different types of stem cells, and introducing them into an early embryo between three to five days of development. We then transfer that embryo to a surrogate female and then harvest the embryos back at day 28 of development, at which point most of the organs are pre-formed.
The human cells will contribute to every organ. But in trying to do that, they will compete with the host organism. Since this is happening inside a pig embryo, which is inside a pig foster mother, the pig cells will win that competition for every organ.
Because you're not putting in enough human cells?
No, because it's a pig environment. Everything is pig. The host, basically, is in control. That's what we see when we do rat mice, or mouse rat: the host always wins the battle.
But we need human cells in the early development -- a few, but not too few -- so that when an organ needs to form, like a pancreas (which develops at around day 25), the pig cells will not respond to that, but if there are human cells in that location, [those human cells] can respond to pancreas formation.
From the work in mice and rats, we know we need some kind of global contribution across multiple tissues -- even a 1% contribution will be sufficient. But if the cells are not there, then they're not going to contribute to that organ. The way we target the specific organ is by removing the competition for that organ.
So if you want it to grow a pancreas, you use an embryo that is not going to grow a pancreas of its own. But you can't control where the other cells go. For instance, you don't want them going to the animal's brain – or its gonads –right?
You don't want the cells to confer any human characteristics in the animal. But even if cells go to the brain, it's not going to confer on the animal human characteristics. A few human cells, even if they're in the brain, won't make it a human brain. Too many cells, that may be a problem, because we do not know what that threshold is.
The objective of our research right now is to look at just 28 days of embryonic development and evaluate what's going on: Are the human cells there? How many? Do they go to the brain? If so, how many? Is this a problem, or is it not a problem? If we find that too many human cells go to the brain, that will probably mean that we wouldn't continue with this approach. At this point, we're not controlling it; we're analyzing it.
"By keeping our research in a very early stage of development, we're not creating a human or a humanoid or anything in between."
What other ethical concerns have arisen?
Conferring human properties to the organism, that is a major concern. I wouldn't like to be involved in that, and so that's what we're trying to assess. By keeping our research in a very early stage of development, we're not creating a human or a humanoid or anything in between.
What specifically sets off the ethical alarms? An animal developing human traits?
Animals developing human characteristics goes beyond what would be considered acceptable. I share that concern. But so far, what we have observed, primarily in rats and mice, is that the host animal dictates development. When you put mouse cells into a rat -- and they're so closely related, sometimes the mouse cells contribute to about 30 percent of the cells in the animal -- the outcome is still a rat. It's the size of a rat. It's the shape of the rat. It has the organ sizes of a rat. Even when the pancreas is fully made out of mouse cells, the pancreas is rat-sized because it grew inside the rat.
This happens even with an organ that is not shared, like a gallbladder, which mice have but rats do not. If you put cells from a mouse into a rat, it never grows a gallbladder. And if you put rat cells into the mouse, the rat cells can end up in the gallbladder even though those rat cells would never have made a gallbladder in a rat.
That means the cell structure is following the directions of the embryo, in terms of how they're going to form and what they're going to make. Based on those observations, if you put human cells into a sheep, we are going to get a sheep with human cells. The organs, the pancreas, in our case, will be the size and shape of the sheep pancreas, but it will be loaded with human cells identical to those of the patient that provided the cells used to generate the stem cells.
But, yeah, if by doing this, the animal acquires the functional or anatomical characteristics associated with a human, it would not be acceptable for me.
So you think these concerns are justified?
Absolutely. They need to be considered. But sometimes by raising these concerns, we prevent technologies from being developed. We need to consider the concerns, but we must evaluate them fully, to determine if they are scientifically justified. Because while we must consider the ethics of doing this, we also need to consider the ethics of not doing it. Every day, 22 people in the US die because they don't receive the organ they need to survive. This shortage is not going to be solved by donations, alone. That's clear. And when people die of old age, their organs are not good anymore.
Since organ transplantation has been so successful, the number of people needing organs has just been growing. The number of organs available has also grown but at a much slower pace. We need to find an alternative, and I think growing the organs in animals is one of those alternatives.
Right now, there's a moratorium on National Institutes of Health funding?
Yes. It's only one agency, but it happens to be the largest biomedical funding source. We have public funding for this work from the California Institute for Regenerative Medicine, and one of my colleagues has funding from the Department of Defense.
"I can say, without NIH funding, it's not going to happen here. It may happen in other places, like China."
Can we put the moratorium in context? How much research in the U.S. is funded by the NIH?
Probably more than 75 percent.
So what kind of impact would lifting that ban have on speeding up possible treatments for those who need a new organ?
Oh, I think it would have a huge impact. The moratorium not only prevents people from seeking funding to advance this area of research, it influences other sources of funding, who think, well, if the NIH isn't doing it, why are we going to do it? It hinders progress.
So with the ban, how long until we can really have organs growing in animals? I've heard five or 10 years.
With or without the ban, I don't think I can give you an accurate estimate.
What we know so far is that human cells don't contribute a lot to the animal embryo. We don't know exactly why. We have a lot of good ideas about things we can test, but we can't move forward right now because we don't have funding -- or we're moving forward but very slowly. We're really just scratching the surface in terms of developing these technologies.
We still need that one major leap in our understanding of how different species interact, and how human cells participate in the development of other species. I cannot predict when we're going to reach that point. I can say, without NIH funding, it's not going to happen here. It may happen in other places, like China, but without NIH funding, it's not going to happen in the U.S.
I think it's important to mention that this is in a very early stage of development and it should not be presented to people who need an organ as something that is possible right now. It's not fair to give false hope to people who are desperate.
So the five to 10 year figure is not realistic.
I think it will take longer than that. If we had a drug right now that we knew could stop heart attacks, it could take five to 10 years just to get it to market. With this, you're talking about a much more complex system. I would say 20 to 25 years. Maybe.
DNA- and RNA-based electronic implants may revolutionize healthcare
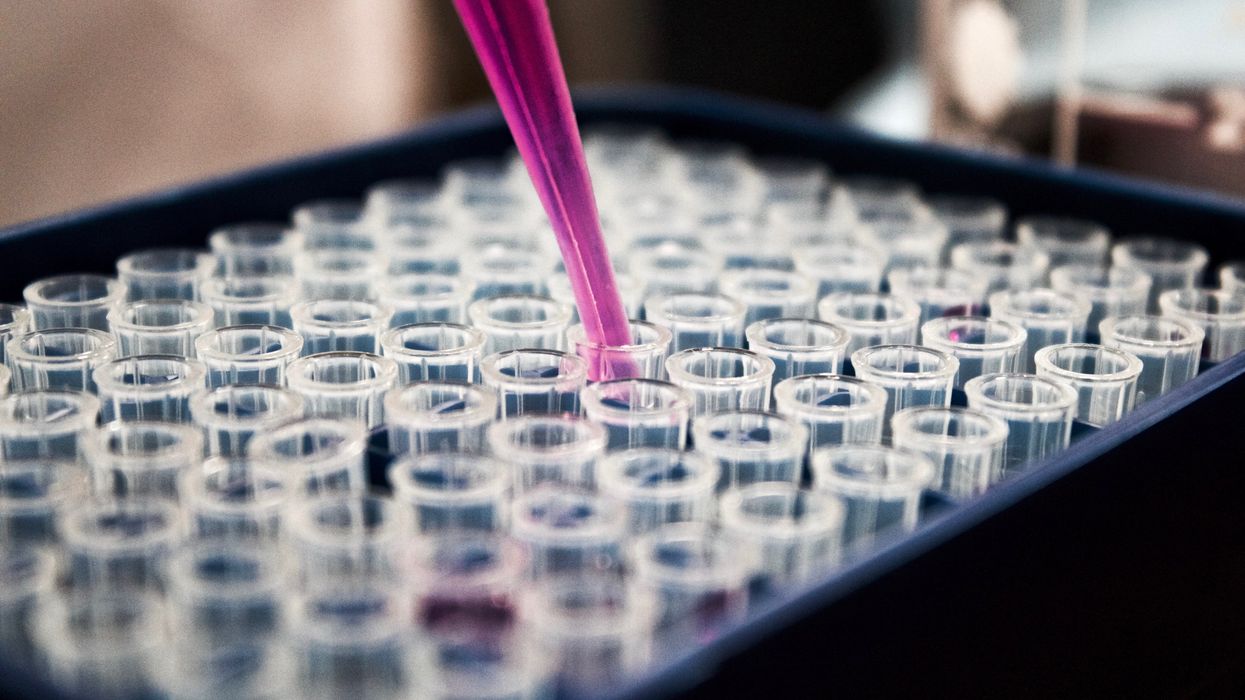
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
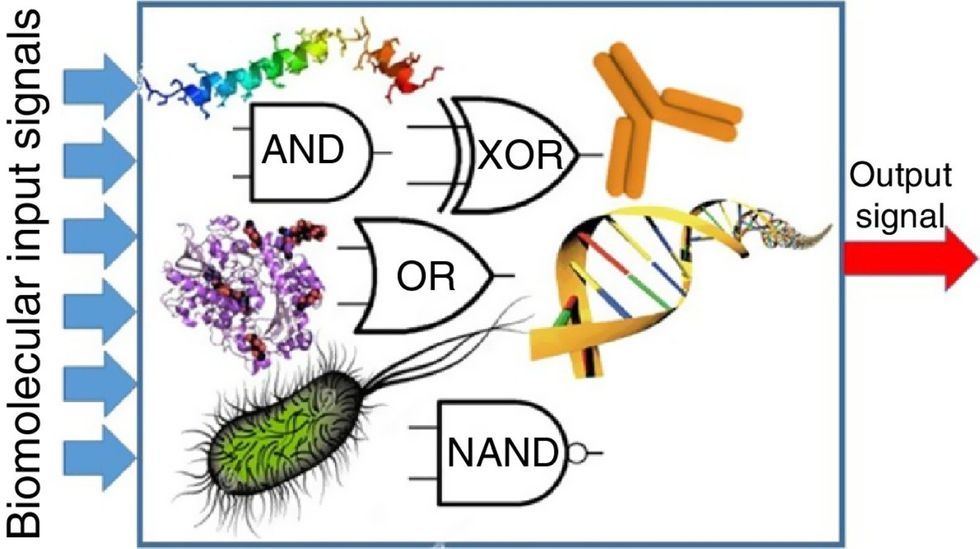
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.