Virtual Reality is Making Medical Care for Kids Less Scary and Painful
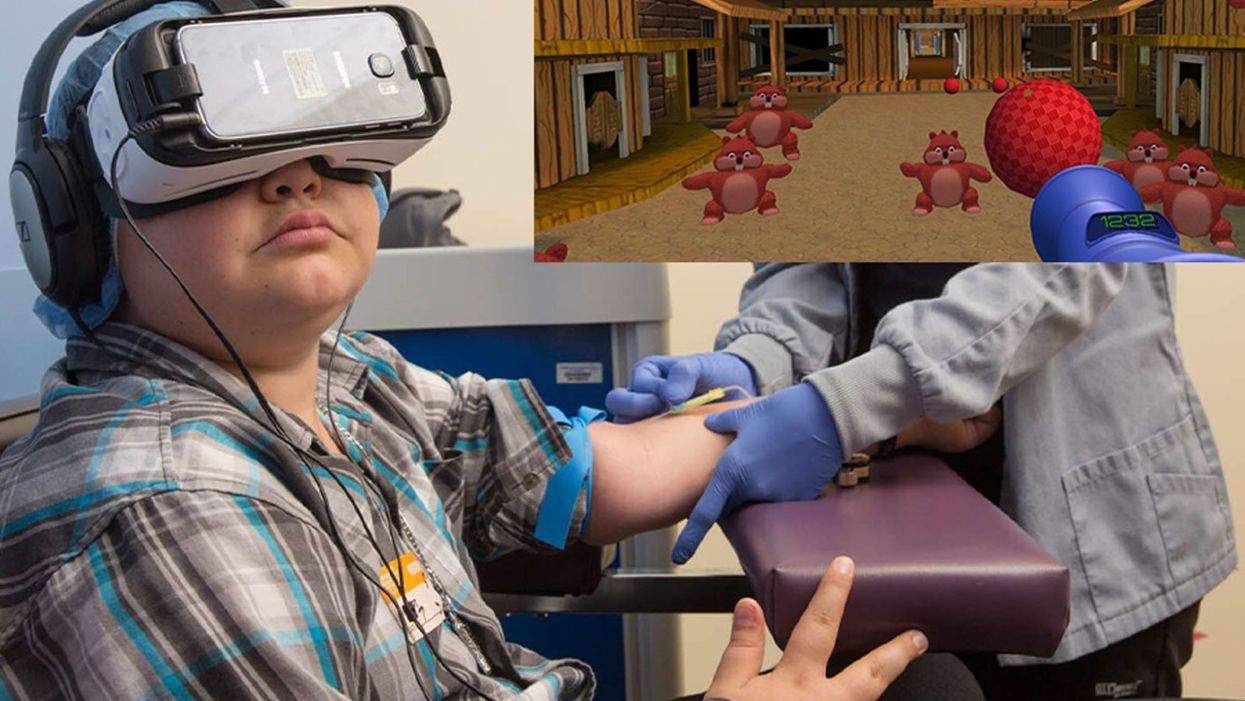
A patient at Children's Hospital Los Angeles (Courtesy of Children's Hospital Los Angeles) plus Bear Blast, developed by AppliedVR.
A blood draw is not normally a fun experience, but these days, virtual reality technology is changing that.
Instead of watching a needle go into his arm, a child wearing a VR headset at Children's Hospital Los Angeles can play a game throwing balls at cartoon bears. In Seattle, at the University of Washington, a burn patient can immerse herself in a soothing snow scene. And at the University of Miami Hospital, a five-minute skin biopsy can become an exciting ride at an amusement park.
VR is transforming once-frightening medical encounters for kids, from blood draws to biopsies to pre-surgical prep, into tolerable ones.
It's literally a game changer, says pediatric neurosurgeon Kurtis Auguste, who uses the tool to help explain pending operations to his young patients and their families. The virtual reality 3-D portrait of their brain is recreated from an MRI, originally to help plan the surgery. The image of normally bland tissue is painted with false colors to better see the boundaries and anomalies of each component. It can be rotated, viewed from every possible angle, zoomed in and out; incisions can be made and likely results anticipated. Auguste has extended its use to patients and families.
"The moment you put these headsets on the kids, we immediately have a link, because honestly, this is how they communicate with each other," says Auguste. "We're all sitting around the table playing games. It's really bridged the distance between me, the pediatric specialist, and my patients" at the Benioff Children's Hospital Oakland, now affiliated with the University of California San Francisco School of Medicine.
The VR experience engages people where they are, immersing them in the environment rather than lecturing them. And it seems to work in all environments, across age and cultural differences, leading to a better grasp of what will be undertaken. That understanding is crucial to meaningful informed consent for surgery. It is particularly relevant for safety-net hospitals, which includes most children's hospitals, because often members of the families were born elsewhere and may have limited understanding of English, not to mention advanced medicine.
Targeting pain
"We're trying to target ways that we can decrease pain, anxiety, fear – what people usually experience as a function of a needle," says Jeffrey Gold, a pioneer in adapting VR at Children's Hospital Los Angeles. He ran the pain clinic there and in 2004 initially focused on phlebotomy, simple blood draws. Many of their kids require frequent blood draws to monitor serious chronic conditions such as diabetes, HIV infection, sickle cell disease, and other conditions that affect the heart, liver, kidneys and other organs.
The scientific explanation of how VR works for pain relief draws upon two basic principles of brain function. The first is "top down inhibition," Gold explains. "We all have the inherent capacity to turn down signals once we determine that signal is no longer harmful, dangerous, hurtful, etc. That's how our brain operates on purpose. It's not just a distraction, it's actually your brain stopping the pain signal at the spinal cord before it can fire all the way up to the frontal lobe."
Second is the analgesic effect from endorphins. "If you're in a gaming environment, and you're having fun and you're laughing and giggling, you are actually releasing endorphins...a neurochemical reaction at the synaptic level of the brain," he says.
Part of what makes VR effective is "what's called a cognitive load, where you have to actually learn something and do something," says Gold. He has worked with developers on a game call Bear Blast, which has proven to be effective in a clinical trial for mitigating pain. But he emphasizes, it is not a one-size-fits all; the programs and patients need to be evaluated to understand what works best for each case.
Gold was a bit surprised to find that VR "actually facilitates quicker blood draws," because the staff doesn't have to manage the kids' anxiety, so "they require fewer needle sticks." The kids, parents, and staff were all having a good time, "and that's a big win when everybody is benefiting." About two years ago the hospital made VR an option that patients can request in the phlebotomy lab, and about half of kids age 4 and older choose to do so.
The technology "gets the kids engaged and performing the activity the way we want them to" to maximize recovery.
VR reduces or eliminates the need to use sedation or anesthesia, which carries a small but real risk of an adverse reaction. And important to parents, it eliminates the recovery time from using sedation, which shortens the visit and time missed from school and work.
A more intriguing question is whether reducing fear and anxiety in early-life experiences with the healthcare system through activities like VR will have a long-term affect on kids' attitudes toward medicine as they grow older. "If you're a screaming meemie when you come get your blood draw when you're five or seven, you're still that anxious adolescent or adult who is all quivering and sweating and avoiding healthcare," Gold says. "That's a longitudinal health outcome I'd love to get my hands on in 10-15 years from now."
Broader applications
Dermatologist Hadar Lev-Tov read about the use of VR to treat pain and decided to try it in his practice at the University of Miami Hospital. He thought, "OK, this is low risk, it's easy to do. So we got some equipment and got it done." It was so affordable he paid for it out of his own pocket, rather than wait to go through administrative channels. The results were so interesting that he decided to publish it as a series of case studies with a wide variety of patients and types of procedures.
Some of them, such as freezing off warts, are not particularly painful. "But there can be a lot of anxiety, especially for kids, which can be worse than pain and can disrupt the procedure." It can trigger a non-rational, primal fight or flight response in the limbic region of the brain.
Adults understand the need for a biopsy of a skin growth and tolerate what might be a momentary flick of pain. "But for a kid you think twice about a biopsy, both because it's a hassle and because it could be a traumatic event for a child," says Lev-Tov. VR has helped to allay such fears and improve medical care.
Integrating VR into practice has been relatively easy, primarily focusing on simple training for staff and ensuring that standard infection control practices are used in handling equipment that is used by different patients. More mundane issues are ensuring that the play back and wi-fi equipment are functioning properly. He has had a few complaints from kids when the procedure is competed and the VR is turned off prematurely, which is why he favors programs like a roller coaster ride that lasts about five minutes, ample time to take a biopsy or two.
The future is today
The pediatric neurosurgeon Auguste is collaborating with colleagues at Oakland Children's to expand use of VR into different areas of care. Cancer specialists often use a port, a bubble installed under the skin in the chest of the child, to administer chemotherapy. But the young patient's curiosity often draws their attention downward to the port and their chin can potentially contaminate or obstruct it, interfering with the procedure. So the team developed a VR game involving birds that requires players to move their heads upward, away from the port, improving administration of the drugs and reducing the risk of infection.
Innovative use of VR just may be one tool that actually makes kids eager to visit the doctor.
Other games are being developed for rehabilitation that require the use of specific nerve and muscle combinations. The technology "gets the kids engaged and performing the activity the way we want them to" to maximize recovery, Auguste explains. "We can monitor their progress by the score on the game, and if it plateaus, maybe switch to another game."
Another project is trying to ease the anxiety and confusion of the patient and family experience within the hospital itself. Hospital staff are creating a personalized VR introductory walking tour that leads from the parking garage through the maze of structures and corridors in the hospital complex to Dr. Auguste's office, phlebotomy, the MRI site, and other locations they might visit. The goal is to make them familiar with key landmarks before they even set foot in the facility. "So when they come the day of the visit they have already taken that exact same path, hopefully more than once."
"They don't miss their MRI appointment and therefore they don't miss their clinical appointment with me," says Auguste. It reduces patient anxiety about the encounter and from the hospital's perspective, it will reduce costs of missed and rescheduled visits simply because patients did not go to the right place at the right time.
The VR visit will be emailed to patients ahead of time and they can watch it on a smartphone installed in a disposable cardboard viewer. Oakland Children's hopes to have the system in place by early next year. Auguste says their goal in using VR, like other health care providers across the country, is "to streamline the entire patient experience."
Innovative use of VR just may be one tool that actually makes kids eager to visit the doctor. That would be a boon to kids, parents, and the health of America.
Meet Dr. Renee Wegrzyn, the first Director of President Biden's new health agency, ARPA-H
Today's podcast guest, Dr. Renee Wegrzyn, directs ARPA-H, a new agency formed last year to spearhead health innovations. Time will tell if ARPA-H will produce advances on the level of its fellow agency, DARPA.
In today’s podcast episode, I talk with Renee Wegrzyn, appointed by President Biden as the first director of a health agency created last year, the Advanced Research Projects Agency for Health, or ARPA-H. It’s inspired by DARPA, the agency that develops innovations for the Defense department and has been credited with hatching world-changing technologies such as ARPANET, which became the internet.
Time will tell if ARPA-H will lead to similar achievements in the realm of health. That’s what President Biden and Congress expect in return for funding ARPA-H at 2.5 billion dollars over three years.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
How will the agency figure out which projects to take on, especially with so many patient advocates for different diseases demanding moonshot funding for rapid progress?
I talked with Dr. Wegrzyn about the opportunities and challenges, what lessons ARPA-H is borrowing from Operation Warp Speed, how she decided on the first ARPA-H project that was announced recently, why a separate agency was needed instead of reforming HHS and the National Institutes of Health to be better at innovation, and how ARPA-H will make progress on disease prevention in addition to treatments for cancer, Alzheimer’s and diabetes, among many other health priorities.
Dr. Wegrzyn’s resume leaves no doubt of her suitability for this role. She was a program manager at DARPA where she focused on applying gene editing and synthetic biology to the goal of improving biosecurity. For her work there, she received the Superior Public Service Medal and, in case that wasn’t enough ARPA experience, she also worked at another ARPA that leads advanced projects in intelligence, called I-ARPA. Before that, she ran technical teams in the private sector working on gene therapies and disease diagnostics, among other areas. She has been a vice president of business development at Gingko Bioworks and headed innovation at Concentric by Gingko. Her training and education includes a PhD and undergraduate degree in applied biology from the Georgia Institute of Technology and she did her postdoc as an Alexander von Humboldt Fellow in Heidelberg, Germany.
Dr. Wegrzyn told me that she’s “in the hot seat.” The pressure is on for ARPA-H especially after the need and potential for health innovation was spot lit by the pandemic and the unprecedented speed of vaccine development. We'll soon find out if ARPA-H can produce gamechangers in health that are equivalent to DARPA’s creation of the internet.
Show links:
ARPA-H - https://arpa-h.gov/
Dr. Wegrzyn profile - https://arpa-h.gov/people/renee-wegrzyn/
Dr. Wegrzyn Twitter - https://twitter.com/rwegrzyn?lang=en
President Biden Announces Dr. Wegrzyn's appointment - https://www.whitehouse.gov/briefing-room/statement...
Leaps.org coverage of ARPA-H - https://leaps.org/arpa/
ARPA-H program for joints to heal themselves - https://arpa-h.gov/news/nitro/ -
ARPA-H virtual talent search - https://arpa-h.gov/news/aco-talent-search/

Dr. Renee Wegrzyn was appointed director of ARPA-H last October.
Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.