Want to Motivate Vaccinations? Message Optimism, Not Doom

There is a lot to be optimistic about regarding the new safe and highly effective vaccines, which are moving society closer toward the goal of close human contact once again.
After COVID-19 was declared a worldwide pandemic by the World Health Organization on March 11, 2020, life as we knew it altered dramatically and millions went into lockdown. Since then, most of the world has had to contend with masks, distancing, ventilation and cycles of lockdowns as surges flare up. Deaths from COVID-19 infection, along with economic and mental health effects from the shutdowns, have been devastating. The need for an ultimate solution -- safe and effective vaccines -- has been paramount.
On November 9, 2020 (just 8 months after the pandemic announcement), the press release for the first effective COVID-19 vaccine from Pfizer/BioNTech was issued, followed by positive announcements regarding the safety and efficacy of five other vaccines from Moderna, University of Oxford/AztraZeneca, Novavax, Johnson and Johnson and Sputnik V. The Moderna and Pfizer vaccines have earned emergency use authorization through the FDA in the United States and are being distributed. We -- after many long months -- are seeing control of the devastating COVID-19 pandemic glimmering into sight.
To be clear, these vaccine candidates for COVID-19, both authorized and not yet authorized, are highly effective and safe. In fact, across all trials and sites, all six vaccines were 100% effective in preventing hospitalizations and death from COVID-19.
All Vaccines' Phase 3 Clinical Data
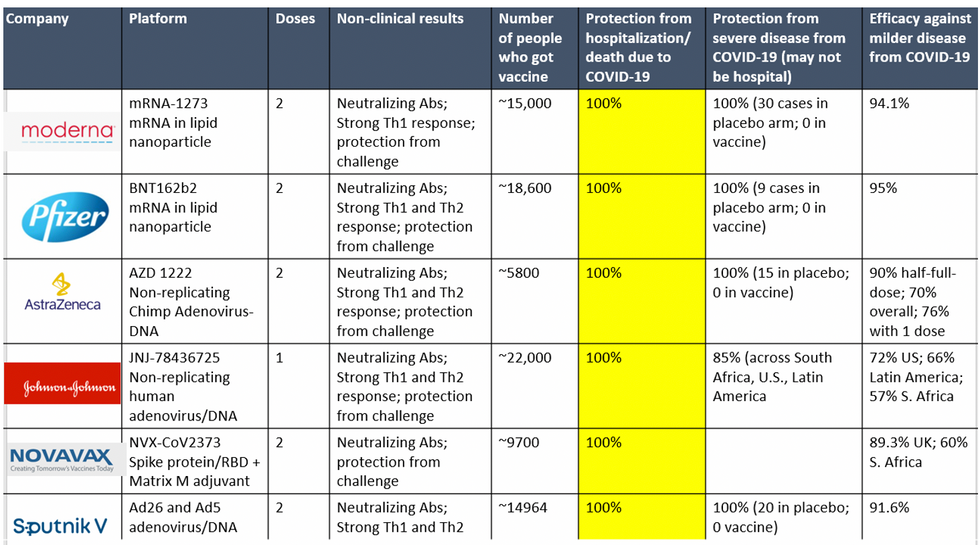
Complete protection against hospitalization and death from COVID-19 exhibited by all vaccines with phase 3 clinical trial data.
This astounding level of protection from SARS-CoV-2 from all vaccine candidates across multiple regions is likely due to robust T cell response from vaccination and will "defang" the virus from the concerns that led to COVID-19 restrictions initially: the ability of the virus to cause severe illness. This is a time of hope and optimism. After the devastating third surge of COVID-19 infections and deaths over the winter, we finally have an opportunity to stem the crisis – if only people readily accept the vaccines.
Amidst these incredible scientific advancements, however, public health officials and politicians have been pushing downright discouraging messaging. The ubiquitous talk of ongoing masks and distancing restrictions without any clear end in sight threatens to dampen uptake of the vaccines. It's imperative that we break down each concern and see if we can revitalize our public health messaging accordingly.
The first concern: we currently do not know if the vaccines block asymptomatic infection as well as symptomatic disease, since none of the phase 3 vaccine trials were set up to answer this question. However, there is biological plausibility that the antibodies and T-cell responses blocking symptomatic disease will also block asymptomatic infection in the nasal passages. IgG immunoglobulins (generated and measured by the vaccine trials) enter the nasal mucosa and systemic vaccinations generate IgA antibodies at mucosal surfaces. Monoclonal antibodies given to outpatients with COVID-19 hasten viral clearance from the airways.
Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other.
Moreover, data from the AztraZeneca trial (including in the phase 3 trial final results manuscript), where weekly self-swabbing was done by participants, and data from the Moderna trial, where a nasal swab was performed prior to the second dose, both showed risk reductions in asymptomatic infection with even a single dose. Finally, real-world data from a large Pfizer-based vaccine campaign in Israel shows a 50% reduction in infections (asymptomatic or symptomatic) after just the first dose.
Therefore, the likelihood of these vaccines blocking asymptomatic carriage, as well as symptomatic disease, is high. Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other. Moreover, as the percentage of vaccinated people increases, it will be increasingly untenable to impose restrictions on this group. Once herd immunity is reached, these restrictions can and should be abandoned altogether.
The second concern translating to "doom and gloom" messaging lately is around the identification of troubling new variants due to enhanced surveillance via viral sequencing. Four major variants circulating at this point (with others described in the past) are the B.1.1.7 variant ("UK variant"), B.1.351 ("South Africa variant), P.1. ("Brazil variant"), and the L452R variant identified in California. Although the UK variant is likely to be more transmissible, as is the South Africa variant, we have no reason to believe that masks, distancing and ventilation are ineffective against these variants.
Moreover, neutralizing antibody titers with the Pfizer and Moderna vaccines do not seem to be significantly reduced against the variants. Finally, although the Novavax 2-dose and Johnson and Johnson (J&J) 1-dose vaccines had lower rates of efficacy against moderate COVID-19 disease in South Africa, their efficacy against severe disease was impressively high. In fact J&J's vaccine still prevented 100% of hospitalizations and death from COVID-19. When combining both hospitalizations/deaths and severe symptoms managed at home, the J&J 1-dose vaccine was 85% protective across all three sites of the trial: the U.S., Latin America (including Brazil), and South Africa.
In South Africa, nearly all cases of COVID-19 (95%) were due to infection with the B.1.351 SARS-CoV-2 variant. Finally, since herd immunity does not rely on maximal immune responses among all individuals in a society, the Moderna/Pfizer/J&J vaccines are all likely to achieve that goal against variants. And thankfully, all of these vaccines can be easily modified to boost specifically against a new variant if needed (indeed, Moderna and Pfizer are already working on boosters against the prominent variants).
The third concern of some public health officials is that people will abandon all restrictions once vaccinated unless overly cautious messages are drilled into them. Indeed, the false idea that if you "give people an inch, they will take a mile" has been misinforming our messaging about mitigation since the beginning of the pandemic. For example, the very phrase "stay at home" with all of its non-applicability for essential workers and single individuals is stigmatizing and unrealistic for many. Instead, the message should have focused on how people can additively reduce their risks under different circumstances.
The public will be more inclined to trust health officials if those officials communicate with nuanced messages backed up by evidence, rather than with broad brushstrokes that shame. Therefore, we should be saying that "vaccinated people can be together with other vaccinated individuals without restrictions but must protect the unvaccinated with masks and distancing." And we can say "unvaccinated individuals should adhere to all current restrictions until vaccinated" without fear of misunderstandings. Indeed, this kind of layered advice has been communicated to people living with HIV and those without HIV for a long time (if you have HIV but partner does not, take these precautions; if both have HIV, you can do this, etc.).
Our heady progress in vaccine development, along with the incredible efficacy results of all of them, is unprecedented. However, we are at risk of undermining such progress if people balk at the vaccine because they don't believe it will make enough of a difference. One of the most critical messages we can deliver right now is that these vaccines will eventually free us from the restrictions of this pandemic. Let's use tiered messaging and clear communication to boost vaccine optimism and uptake, and get us to the goal of close human contact once again.
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained

Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

