When Are We Obligated To Edit Wild Creatures?

Cows on a pasture, who, among other mammals, could experience immense suffering from the New World screwworm.
Combining CRISPR genome editing with the natural phenomenon of gene drive allows us to rewrite the genomes of wild organisms. The benefits of saving children from malaria by editing mosquitoes are obvious and much discussed, but humans aren't the only creatures who suffer. If we gain the power to intervene in a natural world "red in tooth and claw," yet decline to use it, are we morally responsible for the animal suffering that we could have prevented?
Given the power to alter the workings of the natural world, are we morally obligated to use it?
The scenario that may redefine our relationship with the natural world begins with fine clothing. You're dressed to the nines for a formal event, but you arrived early, and it's such a beautiful day that you decided to take a stroll by the nearby lake. Suddenly, you hear the sound of splashing and screams. A child is drowning! Will you dive in to save them? Or let them die, and preserve your expensive outfit?
The philosopher Peter Singer posited this scenario to show that we are all terrible human beings. Just about everyone would save the child and ruin the outfit... leading Singer to question why so few of us give equivalent amounts of money to save children on the other side of the world. The Against Malaria Foundation averages one life saved for every $7000.
But despite having a local bias, our moral compasses aren't completely broken. You never even considered letting the child drown because the situation wasn't your fault. That's because the cause of the problem simply isn't relevant: as the one who could intervene, the consequences are on your head. We are morally responsible for intervening in situations we did not create.
There is a critical difference between Singer's original scenario and the one above: in his version, it was a muddy pond. Any adult can rescue a child from a muddy pond, but a lake is different; you can only save the child if you know how to swim. We only become morally responsible when we acquire the power to intervene.
Few would disagree with either of these moral statements, but when they are combined with increasingly powerful technologies, the implications are deeply unsettling. Given the power to alter the workings of the natural world, are we morally obligated to use it? Recent developments suggest we had best determine the answer soon because, technologically, we are learning to swim. What choices will we make?
Gene drive is a natural phenomenon that occurs when a genetic element reliably spreads through a population even though it reduces the reproductive fitness of individual organisms. Nature has evolved many different mechanisms that result in gene drive, so many that it's nearly impossible to find an organism that doesn't have at least one driving element somewhere in its genome. More than half of our own DNA comprises the broken remnants of gene drives, plus a few active copies.
Scientists have long dreamed of harnessing gene drive to block mosquito-borne disease, with little success. Then came CRISPR genome editing, which works by cutting target genes and replacing them with a new sequence. What happens if you replace the original sequence with the edited version and an encoded copy of the CRISPR system? Gene drive.

CRISPR is a molecular scalpel that we can use to cut, and therefore replace, just about any DNA sequence in any cell. Encode the instructions for the CRISPR system adjacent to the new sequence, and genome editing will occur in the reproductive cells of subsequent generations of heterozygotes, always converting the original wild-type version to the new edited version. By ensuring that offspring will all be born of one sex, or by arranging for organisms that inherit two copies of the gene drive to be sterile, it's theoretically possible to cause a population crash.
(Credit: Esvelt)
When my colleagues and I first described this technology in 2014, we initially focused on the imperative for early transparency. Gene drive research is more like civic governance than traditional technology development: you can decline a treatment recommended by your doctor, but you can’t opt out when people change the shared environment. Applying the traditional closeted model of science to gene drive actively denies people a voice in decisions intended to affect them - and reforming scientific incentives for gene drive could be the first step to making all of science faster and safer.
But open gene drive research is clearly aligned with virtually all of our values. It's when technology places our deepest moral beliefs in conflict that we struggle, and learn who we truly are.
Two of our strongest moral beliefs include our reverence for the natural world and our abhorrence of suffering. Yet some natural species inherently cause tremendous suffering. Are we morally obligated to alter or even eradicate them?
To anyone who doubts that the natural world can inflict unimaginable suffering, consider the New World screwworm.
Judging by history, the answer depends on who is doing the suffering. We view the eradication of smallpox as one of our greatest triumphs, clearly demonstrating that we value human lives over the existence of disease-causing microorganisms. The same principle holds today for malaria: few would argue against using gene drive to crash populations of malarial mosquitoes to help eradicate the disease. There are more than 3500 species of mosquitoes, only three of which would be affected, and once malaria is gone, the mosquitoes could be allowed to recover. It would be extremely surprising if African nations decided not to eradicate malaria.
The more interesting question concerns our moral obligations to animals in the state of nature.
To anyone who doubts that the natural world can inflict unimaginable suffering, consider the New World screwworm, Cochyliomyia hominivorax. Female screwworm flies lay their eggs in open wounds, generating maggots that devour healthy tissue, gluttonously burrowing into the flesh of their host until they drop, engorged and sated, to metamorphose. Yet before they fall, the maggots in a wound emit a pheromone attracting new females, thereby acting as both conductors and performers in a macabre parade that consumes the host alive. The pain is utterly excruciating, so much so that infested people often require morphine before doctors can even examine the wound. Worst of all, the New World screwworm specializes in devouring complex mammals.
Every second of every day, hundreds of millions of animals suffer the excruciating agony of being eaten alive. It has been so throughout North and South America for millions of years. Until 2001, when humanity eradicated the last screwworm fly north of Panama using the “sterile insect technique�. This was not done to protect wild animals or even people, but for economic reasons: the cost of the program was small relative to the immense damage wrought by the screwworm on North American cattle, sheep, and goats. There were no obvious ecological effects. Despite being almost completely unknown even among animal rights activists, the screwworm elimination campaign may well have been one of the greatest triumphs of animal well-being.
Unfortunately, sterile insect technique isn't powerful enough to eradicate the screwworm from South America, where it is more entrenched and protected by the rougher terrain. But gene drive is.

Contrary to news hype, gene drive alone can't cause extinction, but if combined with conventional measures it might be possible to remove targeted species from the wild. For certain species that cause immense suffering, we may be morally obligated to do just that.
(Credit: Esvelt)
South Americans may well decide to eradicate screwworm for the same economic reasons that it was eradicated from North America: the fly inflicts $4 billion in annual damages on struggling rural communities that can least afford it. It need not go extinct, of course; the existence of the sterile insect facility in Panama proves that we can maintain the screwworm indefinitely in captivity on already dead meat.
Yet if for some reason humanity chooses to leave the screwworm as it is - even for upstanding moral reasons, whatever those may be - the knowledge of our responsibility should haunt us.
Tennyson wrote,
Are God and Nature then at strife,
That Nature lends such evil dreams?
So careful of the type she seems,
So careless of the single life.
Evolution by natural selection cares nothing for the single life, nor suffering, nor euphoria, save for their utility in replication. Theoretically, we do. But how much?
[Editor's Note: This story was originally published in May 2018. We are resurfacing archive hits while our staff is on vacation.]
DNA- and RNA-based electronic implants may revolutionize healthcare
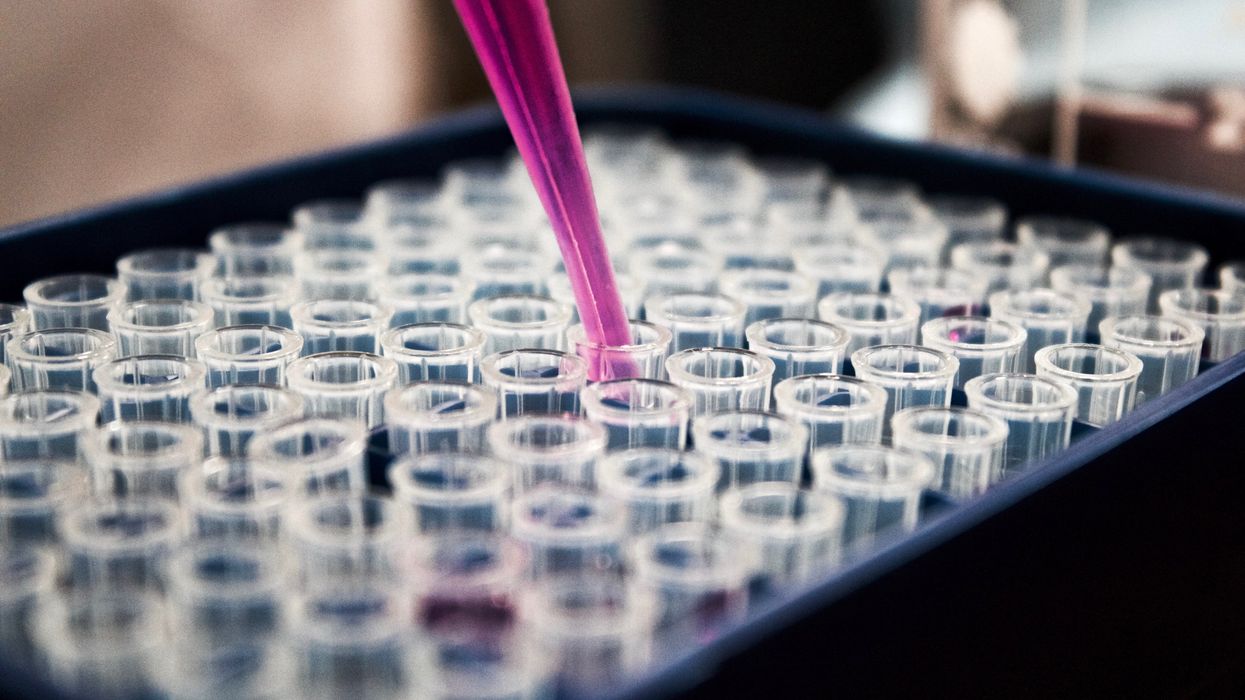
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
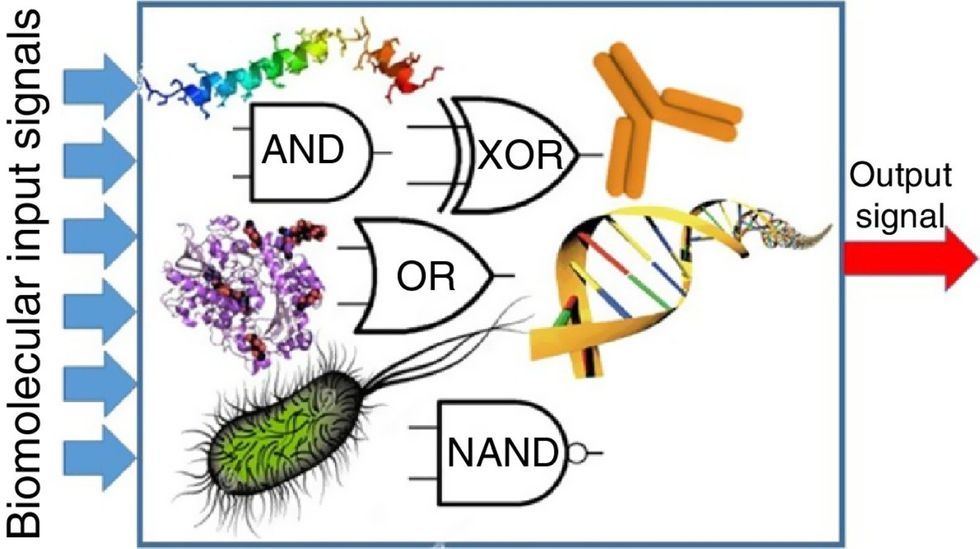
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.