When Are We Obligated To Edit Wild Creatures?

Cows on a pasture, who, among other mammals, could experience immense suffering from the New World screwworm.
Combining CRISPR genome editing with the natural phenomenon of gene drive allows us to rewrite the genomes of wild organisms. The benefits of saving children from malaria by editing mosquitoes are obvious and much discussed, but humans aren't the only creatures who suffer. If we gain the power to intervene in a natural world "red in tooth and claw," yet decline to use it, are we morally responsible for the animal suffering that we could have prevented?
Given the power to alter the workings of the natural world, are we morally obligated to use it?
The scenario that may redefine our relationship with the natural world begins with fine clothing. You're dressed to the nines for a formal event, but you arrived early, and it's such a beautiful day that you decided to take a stroll by the nearby lake. Suddenly, you hear the sound of splashing and screams. A child is drowning! Will you dive in to save them? Or let them die, and preserve your expensive outfit?
The philosopher Peter Singer posited this scenario to show that we are all terrible human beings. Just about everyone would save the child and ruin the outfit... leading Singer to question why so few of us give equivalent amounts of money to save children on the other side of the world. The Against Malaria Foundation averages one life saved for every $7000.
But despite having a local bias, our moral compasses aren't completely broken. You never even considered letting the child drown because the situation wasn't your fault. That's because the cause of the problem simply isn't relevant: as the one who could intervene, the consequences are on your head. We are morally responsible for intervening in situations we did not create.
There is a critical difference between Singer's original scenario and the one above: in his version, it was a muddy pond. Any adult can rescue a child from a muddy pond, but a lake is different; you can only save the child if you know how to swim. We only become morally responsible when we acquire the power to intervene.
Few would disagree with either of these moral statements, but when they are combined with increasingly powerful technologies, the implications are deeply unsettling. Given the power to alter the workings of the natural world, are we morally obligated to use it? Recent developments suggest we had best determine the answer soon because, technologically, we are learning to swim. What choices will we make?
Gene drive is a natural phenomenon that occurs when a genetic element reliably spreads through a population even though it reduces the reproductive fitness of individual organisms. Nature has evolved many different mechanisms that result in gene drive, so many that it's nearly impossible to find an organism that doesn't have at least one driving element somewhere in its genome. More than half of our own DNA comprises the broken remnants of gene drives, plus a few active copies.
Scientists have long dreamed of harnessing gene drive to block mosquito-borne disease, with little success. Then came CRISPR genome editing, which works by cutting target genes and replacing them with a new sequence. What happens if you replace the original sequence with the edited version and an encoded copy of the CRISPR system? Gene drive.

CRISPR is a molecular scalpel that we can use to cut, and therefore replace, just about any DNA sequence in any cell. Encode the instructions for the CRISPR system adjacent to the new sequence, and genome editing will occur in the reproductive cells of subsequent generations of heterozygotes, always converting the original wild-type version to the new edited version. By ensuring that offspring will all be born of one sex, or by arranging for organisms that inherit two copies of the gene drive to be sterile, it's theoretically possible to cause a population crash.
(Credit: Esvelt)
When my colleagues and I first described this technology in 2014, we initially focused on the imperative for early transparency. Gene drive research is more like civic governance than traditional technology development: you can decline a treatment recommended by your doctor, but you can’t opt out when people change the shared environment. Applying the traditional closeted model of science to gene drive actively denies people a voice in decisions intended to affect them - and reforming scientific incentives for gene drive could be the first step to making all of science faster and safer.
But open gene drive research is clearly aligned with virtually all of our values. It's when technology places our deepest moral beliefs in conflict that we struggle, and learn who we truly are.
Two of our strongest moral beliefs include our reverence for the natural world and our abhorrence of suffering. Yet some natural species inherently cause tremendous suffering. Are we morally obligated to alter or even eradicate them?
To anyone who doubts that the natural world can inflict unimaginable suffering, consider the New World screwworm.
Judging by history, the answer depends on who is doing the suffering. We view the eradication of smallpox as one of our greatest triumphs, clearly demonstrating that we value human lives over the existence of disease-causing microorganisms. The same principle holds today for malaria: few would argue against using gene drive to crash populations of malarial mosquitoes to help eradicate the disease. There are more than 3500 species of mosquitoes, only three of which would be affected, and once malaria is gone, the mosquitoes could be allowed to recover. It would be extremely surprising if African nations decided not to eradicate malaria.
The more interesting question concerns our moral obligations to animals in the state of nature.
To anyone who doubts that the natural world can inflict unimaginable suffering, consider the New World screwworm, Cochyliomyia hominivorax. Female screwworm flies lay their eggs in open wounds, generating maggots that devour healthy tissue, gluttonously burrowing into the flesh of their host until they drop, engorged and sated, to metamorphose. Yet before they fall, the maggots in a wound emit a pheromone attracting new females, thereby acting as both conductors and performers in a macabre parade that consumes the host alive. The pain is utterly excruciating, so much so that infested people often require morphine before doctors can even examine the wound. Worst of all, the New World screwworm specializes in devouring complex mammals.
Every second of every day, hundreds of millions of animals suffer the excruciating agony of being eaten alive. It has been so throughout North and South America for millions of years. Until 2001, when humanity eradicated the last screwworm fly north of Panama using the “sterile insect technique�. This was not done to protect wild animals or even people, but for economic reasons: the cost of the program was small relative to the immense damage wrought by the screwworm on North American cattle, sheep, and goats. There were no obvious ecological effects. Despite being almost completely unknown even among animal rights activists, the screwworm elimination campaign may well have been one of the greatest triumphs of animal well-being.
Unfortunately, sterile insect technique isn't powerful enough to eradicate the screwworm from South America, where it is more entrenched and protected by the rougher terrain. But gene drive is.

Contrary to news hype, gene drive alone can't cause extinction, but if combined with conventional measures it might be possible to remove targeted species from the wild. For certain species that cause immense suffering, we may be morally obligated to do just that.
(Credit: Esvelt)
South Americans may well decide to eradicate screwworm for the same economic reasons that it was eradicated from North America: the fly inflicts $4 billion in annual damages on struggling rural communities that can least afford it. It need not go extinct, of course; the existence of the sterile insect facility in Panama proves that we can maintain the screwworm indefinitely in captivity on already dead meat.
Yet if for some reason humanity chooses to leave the screwworm as it is - even for upstanding moral reasons, whatever those may be - the knowledge of our responsibility should haunt us.
Tennyson wrote,
Are God and Nature then at strife,
That Nature lends such evil dreams?
So careful of the type she seems,
So careless of the single life.
Evolution by natural selection cares nothing for the single life, nor suffering, nor euphoria, save for their utility in replication. Theoretically, we do. But how much?
[Editor's Note: This story was originally published in May 2018. We are resurfacing archive hits while our staff is on vacation.]
New tech for prison reform spreads to 11 states
The U.S. has the highest incarceration rate in the world, costing $182 billion per year, partly because its antiquated data systems often fail to identify people who should be released. A tech nonprofit is trying to change that.
A new non-profit called Recidiviz is using data technology to reduce the size of the U.S. criminal justice system. The bi-coastal company (SF and NYC) is currently working with 11 states to improve their systems and, so far, has helped remove nearly 69,000 people — ones left floundering in jail or on parole when they should have been released.
“The root cause is fragmentation,” says Clementine Jacoby, 31, a software engineer who worked at Google before co-founding Recidiviz in 2019. In the 1970s and 80s, the U.S. built a series of disconnected data systems, and this patchwork is still being used by criminal justice authorities today. It requires parole officers to manually calculate release dates, leading to errors in many cases. “[They] have done everything they need to do to earn their release, but they're still stuck in the system,” Jacoby says.
Recidiviz has built a platform that connects the different databases, with the goal of identifying people who are already qualified for release but remain behind bars or on supervision. “Think of Recidiviz like Google Maps,” says Jacoby, who worked on Maps when she was at the tech giant. Google Maps takes in data from different sources – satellite images, street maps, local business data — and organizes it into one easy view. “Recidiviz does something similar with criminal justice data,” Jacoby explains, “making it easy to identify people eligible to come home or to move to less intensive levels of supervision.”
People like Jacoby’s uncle. His experience with incarceration is what inspired her passion for criminal justice reform in the first place.
The problems are vast
The U.S. has the highest incarceration rate in the world — 2 million people according to the watchdog group, Prison Policy Initiative — at a cost of $182 billion a year. The numbers could be a lot lower if not for an array of problems including inaccurate sentencing calculations, flawed algorithms and parole violations laws.
Sentencing miscalculations
To determine eligibility for release, the current system requires corrections officers to check 21 different requirements spread across five different databases for each of the 90 to 100 people under their supervision. These manual calculations are time prohibitive, says Jacoby, and fall victim to human error.
In addition, Recidiviz found that policies aimed at helping to reduce the prison population don’t always work correctly. A key example is time off for good behavior laws that allow inmates to earn one day off for every 30 days of good behavior. Some states' data systems are built to calculate time off as one day per month of good behavior, rather than per day. Over the course of a decade-long sentence, Jacoby says these miscalculations can lead to a huge discrepancy in the calculated release data and the actual release date.
Algorithms
Commercial algorithm-based software systems for risk assessment continue to be widely used in the criminal justice system, even though a 2018 study published in Science Advances exposed their limitations. After the study went viral, it took three years for the Justice Department to issue a report on their own flawed algorithms used to reduce the federal prison population as part of the 2018 First Step Act. The program, it was determined, overestimated the risk of putting inmates of color into early-release programs.
Despite its name, Recidiviz does not build these types of algorithms for predicting recidivism, or whether someone will commit another crime after being released from prison. Rather, Jacoby says the company’s "descriptive analytics” approach is specifically intended to weed out incarceration inequalities and avoid algorithmic pitfalls.
Parole violation laws
Research shows that 350,000 people a year — about a quarter of the total prison population — are sent back not because they’ve committed another crime, but because they’ve broken a specific rule of their probation. “Things that wouldn't send you or I to prison, but would send someone on parole,” such as crossing county lines or being in the presence of alcohol when they shouldn’t be, are inflating the prison population, says Jacoby.
It’s personal for the co-founder and CEO
“I grew up with an uncle who went into the prison system,” Jacoby says. At 19, he was sentenced to ten years in prison for a non-violent crime. A few months after being released from jail, he was sent back for a non-violent parole violation.
“For my family, the fact that one in four prison admissions are driven not by a crime but by someone who's broken a rule on probation and parole was really profound because that happened to my uncle,” Jacoby says. The experience led her to begin studying criminal justice in high school, then college. She continued her dive into how the criminal justice system works as part of her Passion Project while at Google, a program that allows employees to spend 20 percent of their time on pro-bono work. Two colleagues whose family members had also been stuck in the system joined her.
As part of the project, Jacoby interviewed hundreds of people involved in the criminal justice system. “Those on the right, those on the left, agreed that bad data was slowing down reform,” she says. Their research brought them to North Dakota where they began to understand the root of the problem. The corrections department is making “huge, consequential decisions every day [without] … the data,” Jacoby says. In a new video by Recidiviz not yet released, Jacoby recounts her exchange with the state’s director of corrections who told her, “‘It’s not that we have the data and we just don’t know how to make it public; we don’t have the information you think we have.'"
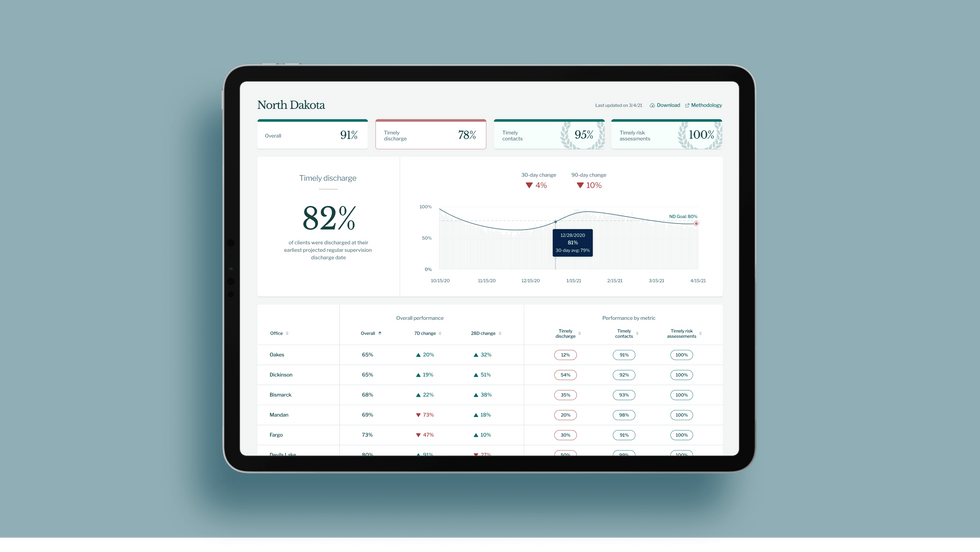
A mock-up (with fake data) of the types of dashboards and insights that Recidiviz provides to state governments.
Recidiviz
As a software engineer, Jacoby says the comment made no sense to her — until she witnessed it first-hand. “We spent a lot of time driving around in cars with corrections directors and parole officers watching them use these incredibly taxing, frankly terrible, old data systems,” Jacoby says.
As they weeded through thousands of files — some computerized, some on paper — they unearthed the consequences of bad data: Hundreds of people in prison well past their release date and thousands more whose release from parole was delayed because of minor paperwork issues. They found individuals stuck in parole because they hadn’t checked one last item off their eligibility list — like simply failing to provide their parole officer with a paystub. And, even when parolees advocated for themselves, the archaic system made it difficult for their parole officers to confirm their eligibility, so they remained in the system. Jacoby and her team also unpacked specific policies that drive racial disparities — such as fines and fees.
The Solution
It’s more than a trivial technical challenge to bring the incomplete, fragmented data onto a 21st century data platform. It takes months for Recidiviz to sift through a state’s information systems to connect databases “with the goal of tracking a person all the way through their journey and find out what’s working for 18- to 25-year-old men, what’s working for new mothers,” explains Jacoby in the video.
TED Talk: How bad data traps people in the U.S. justice system

TED Fellow Clementine Jacoby's TED Talk went live on Jan. 13. It describes how we can fix bad data in the criminal justice system, "bringing thousands of people home, reducing costs and improving public safety along the way."
Clementine Jacoby • TED2022
Ojmarrh Mitchell, an associate professor in the School of Criminology and Criminal Justice at Arizona State University, who is not involved with the company, says what Recidiviz is doing is “remarkable.” His perspective goes beyond academic analysis. In his pre-academic years, Mitchell was a probation officer, working within the framework of the “well known, but invisible” information sharing issues that plague criminal justice departments. The flexibility of Recidiviz’s approach is what makes it especially innovative, he says. “They identify the specific gaps in each jurisdiction and tailor a solution for that jurisdiction.”
On the downside, the process used by Recidiviz is “a bit opaque,” Mitchell says, with few details available on how Recidiviz designs its tools and tracks outcomes. By sharing more information about how its actions lead to progress in a given jurisdiction, Recidiviz could help reformers in other places figure out which programs have the best potential to work well.
The eleven states in which Recidiviz is working include California, Colorado, Maine, Michigan, Missouri, Pennsylvania and Tennessee. And a pilot program launched last year in Idaho, if scaled nationally, with could reduce the number of people in the criminal justice system by a quarter of a million people, Jacoby says. As part of the pilot, rather than relying on manual calculations, Recidiviz is equipping leaders and the probation officers with actionable information with a few clicks of an app that Recidiviz built.
Mitchell is disappointed that there’s even the need for Recidiviz. “This is a problem that government agencies have a responsibility to address,” he says. “But they haven’t.” For one company to come along and fill such a large gap is “remarkable.”
How Leqembi became the biggest news in Alzheimer’s disease in 40 years, and what comes next
Betsy Groves, 73, with her granddaughter. Groves learned in 2021 that she has Alzheimer's disease. She hopes to take Leqembi, a drug approved by the FDA last week.
A few months ago, Betsy Groves traveled less than a mile from her home in Cambridge, Mass. to give a talk to a bunch of scientists. The scientists, who worked for the pharmaceutical companies Biogen and Eisai, wanted to know how she lived her life, how she thought about her future, and what it was like when a doctor’s appointment in 2021 gave her the worst possible news. Groves, 73, has Alzheimer’s disease. She caught it early, through a lumbar puncture that showed evidence of amyloid, an Alzheimer’s hallmark, in her cerebrospinal fluid. As a way of dealing with her diagnosis, she joined the Alzheimer’s Association’s National Early-Stage Advisory Board, which helped her shift into seeing her diagnosis as something she could use to help others.
After her talk, Groves stayed for lunch with the scientists, who were eager to put a face to their work. Biogen and Eisai were about to release the first drug to successfully combat Alzheimer’s in 40 years of experimental disaster. Their drug, which is known by the scientific name lecanemab and the marketing name Leqembi, was granted accelerated approval by the U.S. Food and Drug Administration last Friday, Jan. 6, after a study in 1,800 people showed that it reduced cognitive decline by 27 percent over 18 months.
It is no exaggeration to say that this result is a huge deal. The field of Alzheimer’s drug development has been absolutely littered with failures. Almost everything researchers have tried has tanked in clinical trials. “Most of the things that we've done have proven not to be effective, and it's not because we haven’t been taking a ton of shots at goal,” says Anton Porsteinsson, director of the University of Rochester Alzheimer's Disease Care, Research, and Education Program, who worked on the lecanemab trial. “I think it's fair to say you don't survive in this field unless you're an eternal optimist.”
As far back as 1984, a cure looked like it was within reach: Scientists discovered that the sticky plaques that develop in the brains of those who have Alzheimer’s are made up of a protein fragment called beta-amyloid. Buildup of beta-amyloid seemed to be sufficient to disrupt communication between, and eventually kill, memory cells. If that was true, then the cure should be straightforward: Stop the buildup of beta-amyloid; stop the Alzheimer’s disease.
It wasn’t so simple. Over the next 38 years, hundreds of drugs designed either to interfere with the production of abnormal amyloid or to clear it from the brain flamed out in trials. It got so bad that neuroscience drug divisions at major pharmaceutical companies (AstraZeneca, Pfizer, Bristol-Myers, GSK, Amgen) closed one by one, leaving the field to smaller, scrappier companies, like Cambridge-based Biogen and Tokyo-based Eisai. Some scientists began to dismiss the amyloid hypothesis altogether: If this protein fragment was so important to the disease, why didn’t ridding the brain of it do anything for patients? There was another abnormal protein that showed up in the brains of Alzheimer’s patients, called tau. Some researchers defected to the tau camp, or came to believe the proteins caused damage in combination.
The situation came to a head in 2021, when the FDA granted provisional approval to a drug called aducanumab, marketed as Aduhelm, against the advice of its own advisory council. The approval was based on proof that Aduhelm reduced beta-amyloid in the brain, even though one research trial showed it had no effect on people’s symptoms or daily life. Aduhelm could also cause serious side effects, like brain swelling and amyloid related imaging abnormalities (known as ARIA, these are basically micro-bleeds that appear on MRI scans). Without a clear benefit to memory loss that would make these risks worth it, Medicare refused to pay for Aduhelm among the general population. Two congressional committees launched an investigation into the drug’s approval, citing corporate greed, lapses in protocol, and an unjustifiably high price. (Aduhelm was also produced by the pharmaceutical company Biogen.)
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world.
So far, Leqembi is like Aduhelm in that it has been given accelerated approval only for its ability to remove amyloid from the brain. Both are monoclonal antibodies that direct the immune system to attack and clear dysfunctional beta-amyloid. The difference is that, while that’s all Aduhelm was ever shown to do, Leqembi’s makers have already asked the FDA to give it full approval – a decision that would increase the likelihood that Medicare will cover it – based on data that show it also improves Alzheimer’s sufferer’s lives. Leqembi targets a different type of amyloid, a soluble version called “protofibrils,” and that appears to change the effect. “It can give individuals and their families three, six months longer to be participating in daily life and living independently,” says Claire Sexton, PhD, senior director of scientific programs & outreach for the Alzheimer's Association. “These types of changes matter for individuals and for their families.”
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. It does not halt or reverse the disease, and people do not get better. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world. It has “a rather small effect,” wrote NIH Alzheimer’s researcher Madhav Thambisetty, MD, PhD, in an email to Leaps.org. “It is unclear how meaningful this difference will be to patients, and it is unlikely that this level of difference will be obvious to a patient (or their caregivers).” Another issue is cost: Leqembi will become available to patients later this month, but Eisai is setting the price at $26,500 per year, meaning that very few patients will be able to afford it unless Medicare chooses to reimburse them for it.
The same side effects that plagued Aduhelm are common in Leqembi treatment as well. In many patients, amyloid doesn’t just accumulate around neurons, it also forms deposits in the walls of blood vessels. Blood vessels that are shot through with amyloid are more brittle. If you infuse a drug that targets amyloid, brittle blood vessels in the brain can develop leakage that results in swelling or bleeds. Most of these come with no symptoms, and are only seen during testing, which is why they are called “imaging abnormalities.” But in situations where patients have multiple diseases or are prescribed incompatible drugs, they can be serious enough to cause death. The three deaths reported from Leqembi treatment (so far) are enough to make Thambisetty wonder “how well the drug may be tolerated in real world clinical practice where patients are likely to be sicker and have multiple other medical conditions in contrast to carefully selected patients in clinical trials.”
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval.
Still, there are reasons to be excited. A successful Alzheimer’s drug can pave the way for combination studies, in which patients try a known effective drug alongside newer, more experimental ones; or preventative studies, which take place years before symptoms occur. It also represents enormous strides in researchers’ understanding of the disease. For example, drug dosages have increased massively—in some cases quadrupling—from the early days of Alzheimer’s research. And patient selection for studies has changed drastically as well. Doctors now know that you’ve got to catch the disease early, through PET-scans or CSF tests for amyloid, if you want any chance of changing its course.
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval. His lab already uses blood tests for different types of amyloid, for different types of tau, and for measures of neuroinflammation, neural damage, and synaptic health, but commercially available versions from companies like C2N, Quest, and Fuji Rebio are likely to hit the market in the next couple of years. “[They are] going to transform the diagnosis of Alzheimer's disease,” Porsteinsson says. “If someone is experiencing memory problems, their physicians will be able to order a blood test that will tell us if this is the result of changes in your brain due to Alzheimer's disease. It will ultimately make it much easier to identify people at a very early stage of the disease, where they are most likely to benefit from treatment.”
Learn more about new blood tests to detect Alzheimer's
Early detection can help patients for more philosophical reasons as well. Betsy Groves credits finding her Alzheimer’s early with giving her the space to understand and process the changes that were happening to her before they got so bad that she couldn’t. She has been able to update her legal documents and, through her role on the Advisory Group, help the Alzheimer’s Association with developing its programs and support services for people in the early stages of the disease. She still drives, and because she and her husband love to travel, they are hoping to get out of grey, rainy Cambridge and off to Texas or Arizona this spring.
Because her Alzheimer’s disease involves amyloid deposits (a “substantial portion” do not, says Claire Sexton, which is an additional complication for research), and has not yet reached an advanced stage, Groves may be a good candidate to try Leqembi. She says she’d welcome the opportunity to take it. If she can get access, Groves hopes the drug will give her more days to be fully functioning with her husband, daughters, and three grandchildren. Mostly, she avoids thinking about what the latter stages of Alzheimer’s might be like, but she knows the time will come when it will be her reality. “So whatever lecanemab can do to extend my more productive ways of engaging with relationships in the world,” she says. “I'll take that in a minute.”

