When doctors couldn’t stop her daughter’s seizures, this mom earned a PhD and found a treatment herself.

Savannah Salazar (left) and her mother, Tracy Dixon-Salazaar, who earned a PhD in neurobiology in the quest for a treatment of her daughter's seizure disorder.
Twenty-eight years ago, Tracy Dixon-Salazaar woke to the sound of her daughter, two-year-old Savannah, in the midst of a medical emergency.
“I entered [Savannah’s room] to see her tiny little body jerking about violently in her bed,” Tracy said in an interview. “I thought she was choking.” When she and her husband frantically called 911, the paramedic told them it was likely that Savannah had had a seizure—a term neither Tracy nor her husband had ever heard before.
Over the next several years, Savannah’s seizures continued and worsened. By age five Savannah was having seizures dozens of times each day, and her parents noticed significant developmental delays. Savannah was unable to use the restroom and functioned more like a toddler than a five-year-old.
Doctors were mystified: Tracy and her husband had no family history of seizures, and there was no event—such as an injury or infection—that could have caused them. Doctors were also confused as to why Savannah’s seizures were happening so frequently despite trying different seizure medications.
Doctors eventually diagnosed Savannah with Lennox-Gaustaut Syndrome, or LGS, an epilepsy disorder with no cure and a poor prognosis. People with LGS are often resistant to several kinds of anti-seizure medications, and often suffer from developmental delays and behavioral problems. People with LGS also have a higher chance of injury as well as a higher chance of sudden unexpected death (SUDEP) due to the frequent seizures. In about 70 percent of cases, LGS has an identifiable cause such as a brain injury or genetic syndrome. In about 30 percent of cases, however, the cause is unknown.
Watching her daughter struggle through repeated seizures was devastating to Tracy and the rest of the family.
“This disease, it comes into your life. It’s uninvited. It’s unannounced and it takes over every aspect of your daily life,” said Tracy in an interview with Today.com. “Plus it’s attacking the thing that is most precious to you—your kid.”
Desperate to find some answers, Tracy began combing the medical literature for information about epilepsy and LGS. She enrolled in college courses to better understand the papers she was reading.
“Ironically, I thought I needed to go to college to take English classes to understand these papers—but soon learned it wasn’t English classes I needed, It was science,” Tracy said. When she took her first college science course, Tracy says, she “fell in love with the subject.”
Tracy was now a caregiver to Savannah, who continued to have hundreds of seizures a month, as well as a full-time student, studying late into the night and while her kids were at school, using classwork as “an outlet for the pain.”
“I couldn’t help my daughter,” Tracy said. “Studying was something I could do.”
Twelve years later, Tracy had earned a PhD in neurobiology.
After her post-doctoral training, Tracy started working at a lab that explored the genetics of epilepsy. Savannah’s doctors hadn’t found a genetic cause for her seizures, so Tracy decided to sequence her genome again to check for other abnormalities—and what she found was life-changing.
Tracy discovered that Savannah had a calcium channel mutation, meaning that too much calcium was passing through Savannah’s neural pathways, leading to seizures. The information made sense to Tracy: Anti-seizure medications often leech calcium from a person’s bones. When doctors had prescribed Savannah calcium supplements in the past to counteract these effects, her seizures had gotten worse every time she took the medication. Tracy took her discovery to Savannah’s doctor, who agreed to prescribe her a calcium blocker.
The change in Savannah was almost immediate.
Within two weeks, Savannah’s seizures had decreased by 95 percent. Once on a daily seven-drug regimen, she was soon weaned to just four, and then three. Amazingly, Tracy started to notice changes in Savannah’s personality and development, too.
“She just exploded in her personality and her talking and her walking and her potty training and oh my gosh she is just so sassy,” Tracy said in an interview.
Since starting the calcium blocker eleven years ago, Savannah has continued to make enormous strides. Though still unable to read or write, Savannah enjoys puzzles and social media. She’s “obsessed” with boys, says Tracy. And while Tracy suspects she’ll never be able to live independently, she and her daughter can now share more “normal” moments—something she never anticipated at the start of Savannah’s journey with LGS. While preparing for an event, Savannah helped Tracy get ready.
“We picked out a dress and it was the first time in our lives that we did something normal as a mother and a daughter,” she said. “It was pretty cool.”
“Synthetic Embryos”: The Wrong Term For Important New Research
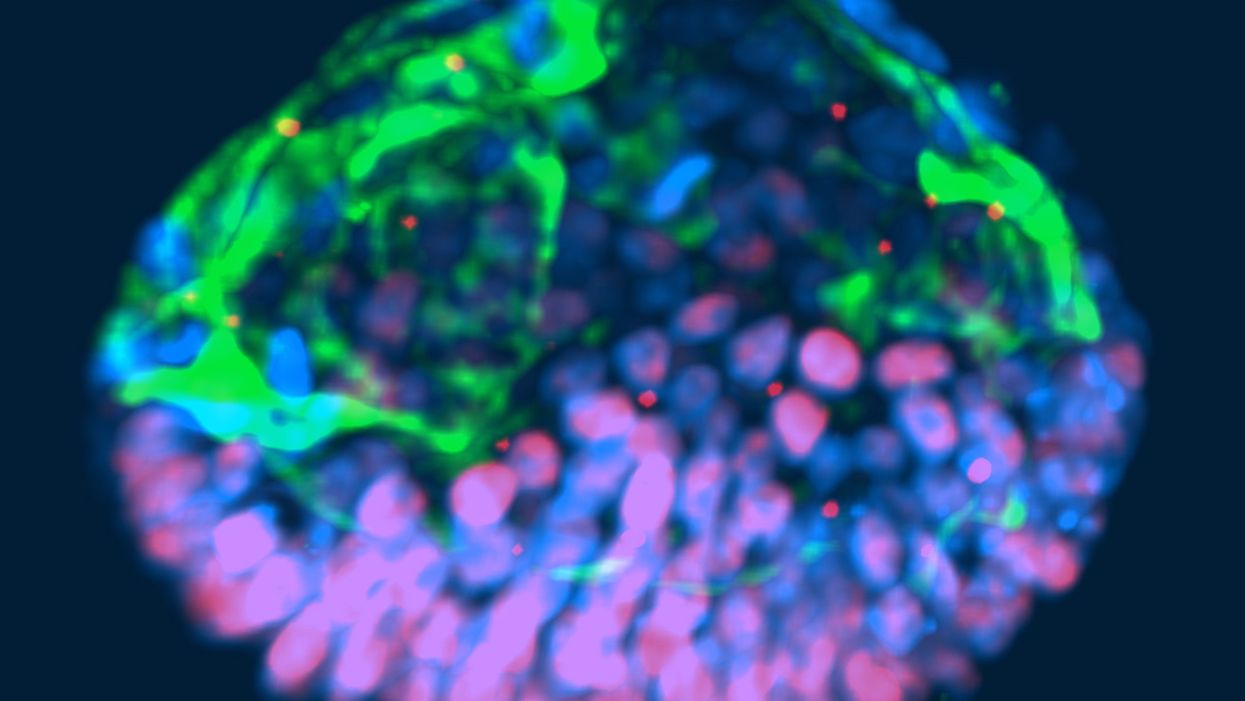
This fluorescent image shows a representative post-implantation amniotic sac embroid.
As a subject of research, an unusual degree of consensus appears to exist among scientists, politicians and the public about human embryos being deserving of special considerations. But what those special considerations should be is less clear. And this is where the subject becomes contentious and opinions diverge because, somewhat surprisingly, what really represents a human embryo has so far not been resolved.
"Prior to implantation, embryos must be given a different level of reverence than after implantation."
In 2002, Howard W. Jones Jr., widely considered the "father" of in vitro fertilization (IVF) in the U.S., argued in a widely acclaimed article titled "What is an embryo?" that a precondition for the definition of a human embryo was successful implantation. Only once implantation established a biological unit between embryo and mother, could a relatively small number of human cells be considered a human embryo.
Because he felt strongly that human embryos, indeed, deserve special considerations, and should receive those during IVF, he pointed out that, even inside a woman's body, most human embryos (in contrast to other species) never implant and, therefore, are never given a chance at human life. Consequently, he reasoned that prior to implantation, embryos must be given a different level of reverence than after implantation.
"One cannot help but wonder about the fog of misconceptions and misrepresentations that still surrounds what an embryo is."
This difference, he felt, should also be reflected in scientific language, proposing that embryos prior to implantation in daily IVF practice be called "pre-embryos," with the term "embryo" reserved for post-implantation-stage embryos. Then still unknown to Jones, recent research findings support this viewpoint, since genetic profiles of pre- and post-implantation stage embryos greatly differ.
In an analogy to nature, which in humans allows implantation of only a small minority of naturally generated pre-embryos, IVF centers around the world routinely discard large numbers of pre-embryos, judged inadequate for producing normal pregnancies. Jones' suggestion that only post-implantation embryos should be considered embryos deserving of special considerations, therefore, not only appears prescient and considerate of current IVF practices, but grounded in scientific reality. One, therefore, cannot help but wonder about the fog of misconceptions and misrepresentations that still surrounds what an embryo is.
"Much of the regulatory environment surrounding research on human embryos is guided by emotions rather than science and logical thinking."
In 1984, a British ethics committee issued the Warnock Report, which still today prohibits scientists worldwide from studying human embryos in a lab beyond 14 days from fertilization or past formation of the so-called primitive streak, whichever comes first. Well-meaning in its day, its intent was to apply special considerations to human pre-embryos by protecting them from the potential of "feeling pain," once the primitive streak arose on day-15 of development. Formation of the primitive streak signifies a process known as gastrulation, when a subset of cells from the inner cell mass of the pre-embryo are transformed into the three germ layers that comprise all tissues of the developing embryo: The ectoderm, which gives rise to the nervous system; the mesoderm, which gives rise to the circulatory system, muscle, and kidneys; and the endoderm which gives rise to the interior lining of the digestive and respiratory tracts, among other tissues.
That pre-embryos may feel pain at that stage of development was far-fetched in 1984; in view of what we have learned about early human embryology in the 33 years since, it remains untenable today. And, yet, scientists all over the world remain bound by the ethical constraints imposed by the Warnock Report.
A similar ethical paradox exists today for guidelines affecting huge numbers of so-called "abandoned" cryopreserved embryos, often stored ad infinitum in IVF centers all over the world. These are pre-embryos, whose "parents" are no longer responsive to queries from their IVF centers. Current U.S. guidelines allow the disposal of such pre-embryos but prohibit their use in research that may benefit mankind. One, however, wonders whether disposal of huge numbers of abandoned embryos is really more ethical than their use in potentially life-saving human research?
That much of the regulatory environment surrounding research on human embryos is, indeed, guided by emotions rather than science and logical thinking, is also demonstrated by recently expressed concern about so-called "artificial" or "synthetic" embryos. Though both of these terms suggest impending ability to create human embryos from synthetic building blocks, this is not what these terms are meant to describe (such abilities also are not on the horizon). They also do not describe abilities to create gametes (i.e., eggs and sperm) from somatic cells by reprogramming adult peripheral cells, which has already been successfully done in mice by Japanese investigators, leading to the creation of healthy embryos and births and three generations of healthy pubs. Such an approach is at least conceivable as an upcoming infertility treatment.
"A team of biologists and engineers at the University of Michigan recently received media attention after creating organoids from embryonic stem cells that resembled human embryos."
What all of this noise is really about is the discovery that, as several Rockefeller University investigators recently noted, "Cells have an intrinsic ability to self-assemble and self-organize into complex and functional tissues and organs." Investigators have taken advantage of this ability by creating in the lab so-called "organoids" from accumulations of individual embryonic stem cells. They are defined by three characteristics: (i) they contain a variety of cell types and tissue layers, all typical for a given organ; (ii) these cells are organized similarly to their organization in a specific organ; and (iii) the organoid mimics functions of the organ.
Several other biologists from the Cincinnati Children Hospital Medical Center recently noted that in the last five years, quite a variety of human stem cell-derived organoids, including all three germ layers, have been generated by different research groups around the world, thereby establishing new human model systems that can be used outside the body, in a dish, to investigate otherwise difficult-to-approach organs. Interestingly, they can also be used to investigate early stages of human embryological development.
A team of biologists and engineers at the University of Michigan recently received media attention after creating organoids from embryonic stem cells that resembled human embryos and, therefore, were given the name "embroids." Though clearly not embryos (the only thing they had in common with human embryos were cell types), they were nevertheless awarded in at least one article the identity of "artificial embryos," which "no one knows how to handle." As Howard Jones so correctly noted, with the word embryo often comes undeserved reverence.
"Any association with the term "embryo" should be avoided; it is not only misleading and irresponsible but scientifically incorrect."
Artificial embryos, therefore, do not exist. Organoids that resemble embryos (i.e., "embroids"), while potentially very useful research objects in studies of early human embryonic cell organization and lineage development, are not embryos--not even pre-embryos. Special considerations for "artificial" or "synthetic" embryos, as recently advocated by some scientists, therefore, appear ethically undeserved. How misdirected and forced some of these efforts are is probably best demonstrated by a recent publication in which a group of Harvard University investigators proposed the term "synthetic human entities with embryo-like features" or SHEEFS" in place of "organoids." Preferably, however, in describing these laboratory-created entities, any association with the term "embryo" should be avoided. It is not only misleading and irresponsible but scientifically incorrect.
Clinical reproductive medicine and reproductive biology, for valid ethical reasons, but also because of myths, misperceptions and, sometimes, outright misrepresentations of facts for political reasons, are under more public scrutiny than most other science areas. Yet, at least in the realm of biomedical research, nothing appears more important than better understanding the first few days of human embryo development. A recent study involving genetic editing of human embryos, reported by British investigators in Nature, once again confirmed what biologist have known for some time: No animal model faithfully recapitulates most of human developmental origins. The most important secrets nature still has to tell us, will not be revealed through mouse or other animal studies. We will discover them only through the study of early-stage human embryos – and we, therefore, should not limit the use of lab-grown organoids to help further that research.
Understanding early human development "will not only greatly enhance the biological understanding of our species; but also will open groundbreaking new therapeutic options in all areas of medicine."
As Howard Jones intuitively noticed, words matter. Appropriate and uniformly accepted definitions and terms are not only essential for scientific communications but, within the context of human reproduction, often elicit strong emotional reactions, and are easily misappropriated by those opposed to most interventions into human reproduction.
Who does not recall the early days of IVF in the late 1970s, when even reputable news outlets raised the specter of Frankenstein monsters created through the IVF process? Millions of IVF births later, a Nobel Prize in Medicine and Physiology was in 2010 finally awarded to the biologist Robert Edwards who, together with the gynecologist Patrick Steptoe, reported the first live birth through IVF on July 25, 1978. Many more awards are still waiting for recipients who through the study of early human embryo development will discover how cell fate is determined and cells acquire highly specific functions; how rapid cell proliferation takes place and, when required, stops; why chromosomal abnormalities are so common in early stage embryos and what their function may be.
Those who will discover these and many other important answers, will not only greatly enhance the biological understanding of our species; but also will open groundbreaking new therapeutic options in all areas of medicine. Learning how to control cell proliferation, for example, will likely revolutionize cancer therapy; I started my research career in biology with a study published in 1980 of "common denominators of pregnancy and malignancy." If regulatory prohibitions are not allowed to interfere in rapidly progressing research opportunities involving organoids and pre-embryos, we will, finally, see the circle closing, with the most rewarding benefits for mankind ever achieved through biological research.
Editor's Note: Read a different viewpoint here written by one of the world's top experts on the ethics of stem cell research.
We Should Resist Making “Synthetic Embryos” Too Realistic
A rendering of emerging medical technology.
Ethics needs context. So does science – specifically, science that aims to create bioengineered models of early human embryo development in a dish (hereafter synthetic embryos). Even the term "synthetic embryos" begs for an explanation. What are these? And why would anyone want to create them?
"This knowledge may help scientists understand how certain birth defects are formed and why miscarriages often occur."
First the research context. Synthetic embryos are stem cell-derived simulations of human post-implantation embryos that are designed to mimic a stage of early development called gastrulation. That's the stage—around 14-15 days after fertilization – when embryos begin to form a very primitive body plan (basic dorsal-ventral and anterior-posterior axes, and distinct cell lineages). Researchers are starting to create synthetic embryos in the lab – albeit imperfect and incomplete versions – to learn how gastrulation might unfold in real human embryos embedded unseen in the womb. This knowledge may help scientists understand how certain birth defects are formed and why miscarriages often occur soon after implantation. As such, synthetic embryos are meant to be models of human embryo development, not themselves actually embryos. But will synthetic embryos ever get to the point where they are practically the same thing as "natural" human embryos? That is my concern and why I think researchers should avoid creating synthetic embryos capable of doing everything natural embryos can do.
It may not be too difficult to prevent this slide from synthetic to real. Synthetic embryos must be created using sophisticated 3D culture systems that mimic the complex architecture of human embryos. These complex culture systems also have to incorporate precise microinjection systems to chemically trigger the symmetry-breaking events involved in early body plan formation. In short, synthetic embryos need a heavy dose of engineering to get their biological processes going and to help keep them going. And like most engineered entities, designs can be built into the system early to serve well-considered goals – in our case, the goal of not wanting to create synthetic embryos that are too realistic.
"If one wants to study how car engines work, one can model an engine without also modeling the wheels, transmission, and every other car part together."
A good example of this point is found a report published in Nature Communications where scientists created a human stem cell-based 3D model that faithfully recapitulates the biological events around post-implantation amniotic sac development. Importantly, however, the embryo model they developed lacked several key structures and therefore – despite its partial resemblance to an early human embryo – did not have complete human form and potential. While fulfilling their model's aim of revealing a previously inaccessible early developmental event, the team intentionally did not recreate the entire post-implantation human embryo because they did not want to provoke any ethical concerns, as the lead author told me personally. Besides, creating a complete synthetic embryo was not necessary or scientifically justified for the research question they were pursuing. This example goes to show that researchers can create a synthetic embryo to model specific developmental events they want to study without modeling every aspect of a developing embryo. Likewise – to use a somewhat imprecise but instructive analogy – if one wants to study how car engines work, one can model an engine without also modeling the wheels, transmission, and every other car part together.

A representative "synthetic embryo," which in some ways resembles a post-implantation embryo around 14 days after fertilization.
(Courtesy of Yue Shao)
But why should researchers resist creating complete synthetic embryos? To answer this, we need some policy context. Currently there is an embryo research rule in place – a law in many nations, in others a culturally accepted agreement – that intact human embryos must not be grown for research in the lab for longer than 14 consecutive days after fertilization or the formation of the primitive streak (a faint embryonic band that signals the start of gastrulation). This is commonly referred to as the 14-day rule. It was established in the UK decades ago to carve out a space for meritorious human embryo research while simultaneously assuring the public that researchers won't go too far in cultivating embryos to later developmental stages before destroying them at the end of their studies. Many citizens accepting of pre-implantation stage human embryo research would not have tolerated post-implantation stage embryo use. The 14-day rule was a line in the sand, drawn to protect the advancement of embryo research, which otherwise might have been stifled without this clear stopping point. To date, the 14-day rule has not been revoked anywhere in the world, although new research in extended natural embryo cultivation is starting to put some pressure on it.
"Perhaps the day will come when scientists don't have to apply for research funding under such a dark cloud of anti-science sentiment."
Why does this policy context matter? The creation of complete synthetic embryos could raise serious questions (some of them legal) about whether the 14-day rule applies to these lab entities. Although they can be constructed in far fewer than 14 days, they would, at least in theory, be capable of recapitulating all of a natural embryo's developmental events at the gastrulation stage, thus possibly violating the spirit of the 14-day rule. Embryo research laws and policies worldwide are not ready yet to tackle this issue. Furthermore, professional guidelines issued by the International Society for Stem Cell Research prohibit the culture of any "organized embryo-like cellular structures with human organismal potential" to be cultured past the formation of the primitive streak. Thus, researchers should wait until there is greater clarity on this point, or until the 14-day rule is revised through proper policy-making channels to explicitly exclude complete synthetic embryos from its reach.
I should be clear that I am not basing my recommendations on any anti-embryo-research position per se, or on any metaphysical position regarding the positive moral status of synthetic embryos. Rather, I am concerned about the potential backlash that research on complete synthetic embryos might bring to embryo research in general. I began this essay by saying that ethics needs context. The ethics of synthetic embryo research needs to be considered within the context of today's fraught political environment. Perhaps the day will come when scientists don't have to apply for research funding under such a dark cloud of anti-science sentiment. Until then, however, it is my hope that scientists can fulfill their research aims by working on an array of different but each purposefully incomplete synthetic embryo models to generate, in the aggregate of their published work, a unified portrait of human development such that biologically complete synthetic embryo models will not be necessary.
Editor's Note: Read a different viewpoint here written by a leading New York fertility doctor/researcher.