Why Food Allergies Are Surging

A baby who cannot tolerate milk due to an allergy.
Like any life-threatening medical condition that affects children, food allergies can traumatize more than just the patient. My wife and I learned this one summer afternoon when our daughter was three years old.
Emergency room visits for anaphylaxis in children more than doubled from 2010 to 2016.
At an ice cream parlor, I gave Samantha a lick of my pistachio cone; within seconds, red blotches erupted on her skin, her lips began to swell, and she complained that her throat felt funny. We rushed her to the nearest emergency room, where a doctor injected her with epinephrine. Explaining that the reaction, known as anaphylaxis, could have been fatal if left unchecked, he advised us to have her tested for nut allergies—and to start carrying an injector of our own.
After an allergist confirmed Sam's vulnerability to tree nuts and peanuts, we figured that keeping her safe would be relatively simple. But food allergies often come in bunches. Over the next year, she wound up back in the ER after eating bread with sesame seeds at an Italian restaurant, and again after slurping buckwheat noodles at our neighborhood Japanese. She hated eggs, so we discovered that (less severe) allergy only when she vomited after eating a variety of products containing them.
In recent years, a growing number of families have had to grapple with such challenges. An estimated 32 million Americans have food allergies, or nearly 10 percent of the population—10 times the prevalence reported 35 years ago. The severity of symptoms seems to be increasing, too. According to a study released in January by Food Allergy Research & Education (FARE), a Virginia-based nonprofit, insurance claims for anaphylactic food reactions rose 377 percent in the U.S. from 2007 to 2016.
Because food allergies most commonly emerge in childhood, these trends are largely driven by the young. An insurance-industry study found that emergency room visits for anaphylaxis in children more than doubled from 2010 to 2016. Peanut allergies, once rare, tripled in kids between 1997 and 2008. "The first year, it was 1 in 250," says Scott Sicherer, chief of pediatric allergy and immunology at New York City's Mount Sinai Hospital, who led that study. "When we did the next round of research, in 2002, it was 1 in 125. I thought there must be a mistake. But by 2008, it was 1 in 70."
The forces behind these dire statistics—as well as similar numbers throughout the developed world—have yet to be positively identified. But the leading suspects are elements of our modern lifestyle that can throw the immune system out of whack, prompting potentially deadly overreactions to harmless proteins. Although parents can take a few steps that might lessen their children's risk, societal changes may be needed to brighten the larger epidemiological picture.
Meanwhile, scientists are racing to develop therapies that can induce patients' hyped-up immune defenses to chill. And lately, they've made some big strides toward that goal.
A Variety of Culprits
In the United States, about 90 percent of allergic reactions come from eight foods: milk, eggs, peanuts, tree nuts, soy, wheat, fish, and shellfish. The list varies from country to country, depending on dietary customs, but what the trigger foods all have in common is proteins that can survive breakdown in the stomach and enter the bloodstream more or less intact.
"When we were kids, we played in the dirt. Today, children tend to be on their screens, inside sealed buildings."
A food allergy results from a chain of biochemical misunderstandings. The first time the immune system encounters an allergen (as a protein that triggers an allergy is known), it mistakes the substance for a hostile invader—perhaps a parasite with a similar molecular profile. In response, it produces an antibody called immunoglobin E (IgE), which is designed to bind to a specific protein and flag it for attack. These antibodies circulate through the bloodstream and attach to immune-system foot soldiers known as mast cells and basophils, which congregate in the nose, throat, lungs, skin, and gastrointestinal tract.
The next time the person is exposed to the allergen, the IgE antibodies signal the warrior cells to blast the intruder with histamines and other chemical weapons. Tissues in the affected areas swell and leak fluid; blood pressure may fall. Depending on the strength of the reaction, collateral damage to the patient can range from unpleasant—itching, runny nose, nausea—to catastrophic.
This kind of immunological glitchiness runs in families. Genome-wide association studies have identified a dozen genes linked to allergies of all types, and twin studies suggest that about 80 percent of the risk of food allergies is heritable. But why one family member shows symptoms while another doesn't remains unknown. Nor can genetics explain why food allergy rates have skyrocketed in such a brief period. For that, we must turn to the environment.
First, it's important to note that rates of all allergies are rising—including skin and respiratory afflictions—though none as rapidly or with as much risk of anaphylaxis as those involving food. The takeoff was already underway in the late 1980s, when British epidemiologist David P. Strachan found that children in larger households had fewer instances of hay fever. The reason, he suggested, was that their immune systems were strengthened by exposure to their siblings' germs. Since then, other researchers have discerned more evidence for Strachan's "hygiene hypothesis": higher rates of allergy (as well as autoimmune disorders) in cities versus rural areas, in industrialized countries versus developing ones, in lab animals raised under sterile conditions versus those exposed to germs.
Fending off a variety of pathogens, experts theorize, helps train the immune system to better distinguish friend from foe, and to respond to threats in a more nuanced manner. In an era of increasing urbanization, shrinking family sizes, and more sheltered lifestyles, such conditioning may be harder to come by. "When we were kids, we played in the dirt," observes Cathryn R. Nagler, a professor and food allergy researcher at the University of Chicago. "Today, children tend to be on their screens, inside sealed buildings."
But other factors may be driving the allergy epidemic as well. More time indoors, for example, means less exposure to sunlight, which can lead to a deficiency in vitamin D—a nutrient crucial to immune system regulation. The growing popularity of processed foods filled with refined fats and sugars may play a role, along with rising rates of obesity, by promoting tissue inflammation that could increase some people's risk of immunological mayhem. And the surge in allergies also correlates with several trends that may be altering the human microbiome, the community of microbes (including bacteria, viruses, and fungi, among others) that inhabits our guts, skin, and bodily orifices.
The microbiome connection may be particularly relevant to food allergies. In 2014, a team led by Nagler published a landmark study showing that Clostridia, a common class of gut bacteria, protects against these allergies. When the researchers fed peanut allergens to germ-free mice (born and raised in sterile conditions) and to mice treated with antibiotics as newborns (reducing their gut bacteria), the animals showed a strong immunological response. This sensitization could be reversed, however, by reintroducing Clostridia—but not another class of bacteria, Bacteroides—into the mice. Further experiments revealed that Clostridia caused immune cells to produce high levels of interleukin-22 (IL-22), a signaling molecule known to decrease the permeability of the intestinal lining.
"In simple terms," Nagler says, "what we found is that these bacteria prevent food allergens from gaining access to the blood in an intact form that elicits an allergic reaction."
A growing body of evidence suggests that our eating habits are throwing our gut microbiota off-balance, in part by depriving helpful species of the dietary fiber they feed on. Our increasing exposure to antibiotics and antimicrobial compounds may be harming our beneficial bugs as well. These depletions could affect kids from the moment they enter the world: Because babies are seeded with their mothers' microbiota as they pass through the birth canal, they may be inheriting a less diverse microbiome than did previous generations. And the rising rate of caesarian deliveries may be further depriving our children of the bugs they need.
On expert suggests two measures worth a try: increasing consumption of fiber, and reducing use of antimicrobial agents, from antibacterial cleaners to antibiotics.
So which culprit is most responsible for the food allergy upsurge? "The illnesses that we're measuring are complex," says Sicherer. "There are multiple genetic inputs, which interact with one another, and there are multiple environmental inputs, which interact with each other and with the genes. There's not one single thing that's causing this. It's a conglomeration."
What Parents Can Do
For anyone hoping to reduce their child's or their own odds of developing a food allergy (rates of adult onset are also increasing), the current state of science offers few guideposts. As with many other areas of health research, it's hard to know when the data is solid enough to warrant a particular course of action. A case in point: the American Academy of Pediatrics once recommended that children at risk of allergy to peanuts (as evidenced by family history, other food allergies, or eczema) wait to eat them until age three; now, the AAP advises those parents to start their babies at four months, citing epidemiological evidence that early exposure may prevent peanut allergies.
And it's all too easy for a layperson to draw mistaken conclusions from media coverage of such research—inferring, for instance, that taking commercially available probiotics might have a protective effect. Unfortunately, says Nagler, none of those products even contain the relevant kind of bacteria.
Although, as a research scientist, she refrains from giving medical advice, Nagler does suggest (based on a large body of academic literature) that two measures are worth a try: increasing consumption of fiber, and reducing use of antimicrobial agents, from antibacterial cleaners to antibiotics. Yet she acknowledges that it's not always possible to avoid the suspected risk factors for food allergies. Sometimes an antibiotic is a lifesaving necessity, for example—and it's tough to avoid exposure to such drugs altogether, due to their use in animal feed and their consequent presence in many foods and in the water supply. If these chemicals are contributing to the food allergy epidemic, protecting ourselves will require action from farmers, doctors, manufacturers, and policymakers.
My family's experience illustrates the limits of healthy lifestyle choices in mitigating allergy risk. My daughter and son were born without C-sections; both were breastfed as well, receiving maximum microbial seeding from their mother. As a family, we eat exemplary diets, and no one could describe our home as excessively clean. Yet one child can't taste nuts, sesame, or buckwheat without becoming dangerously ill. "You can do everything right and still have allergies," says Ian A. Myles, a staff clinician at the National Institute of Allergy and Infectious Diseases. "You can do everything wrong and not have allergies. The two groups overlap."
The Latest Science Shows Promise
But while preventing all food allergies is clearly unrealistic, researchers are making remarkable progress in developing better treatments—therapies that, instead of combating symptoms after they've started (like epinephrine or antihistamines), aim to make patients less sensitive to allergens in the first place. One promising approach is oral immunotherapy (OIT), in which patients consume small but slowly increasing amounts of an allergen, gradually reducing their sensitivity. A study published last year in the New England Journal of Medicine showed that an experimental OIT called AR101, consisting of a standardized peanut powder mixed into food, enabled 67 percent of participants to tolerate a dose equivalent to two peanut kernels—a potential lifesaver if they were accidentally exposed to the real thing.
Because OIT itself can trigger troublesome reactions in some patients, however, it's not for everyone. Another experimental treatment, sublingual immunotherapy (SLIT) uses an allergen solution or dissolving tablet placed beneath the tongue; although its results are less robust than OIT's, it seems to generate milder side effects. Epicutaneous immunotherapy (EPIT) avoids the mouth entirely, using a technology similar to a nicotine patch to deliver allergens through the skin. Researchers are also exploring the use of medications known as biologics, aiming to speed up the action of immunotherapies by suppressing IgE or targeting other immune-system molecules.
These findings suggest that drugs based on microbial metabolites could help protect vulnerable individuals against a wide range of allergies.
One downside of the immunotherapy approach is that in most cases the allergen must be taken indefinitely to maintain desensitization. To provide a potentially permanent fix, scientists are working on vaccines that use DNA or peptides (protein fragments) from allergens to reset patients' immune systems.
Nagler is attacking the problem from a different angle—one that starts with the microbiome. In a recent study, a follow-up to her peanut-allergy investigation, she and her colleagues found that Clostridia bacteria protect mice against milk allergy as well; they also identified a particular species responsible, known as Anaerostipes caccae. The bugs, the team determined, produce a short-chain fatty acid called butyrate, which modulates many immune activities crucial to maintaining a well-sealed gut.
These findings suggest that drugs based on microbial metabolites could help protect vulnerable individuals against a wide range of allergies. Nagler has launched a company, ClostraBio, to develop biotherapeutics based on this notion; she expects its first product, using synthetic butyrate, to be ready for clinical trials within the next two years.
My daughter could well be a candidate for such a medication. Sam, now 15, is a vibrant, resilient kid who handles her allergies with confidence and humor. Thanks to vigilance and luck (on her part as well as her parents'), she hasn't had another food-related ER visit in more than a decade; she's never had to use her Epi-Pen. Still, she says, she would welcome the arrival of a pill that could reduce the danger. "I've learned how to watch out for myself," she says. "But it would be nice not to have to be so careful."
DNA- and RNA-based electronic implants may revolutionize healthcare
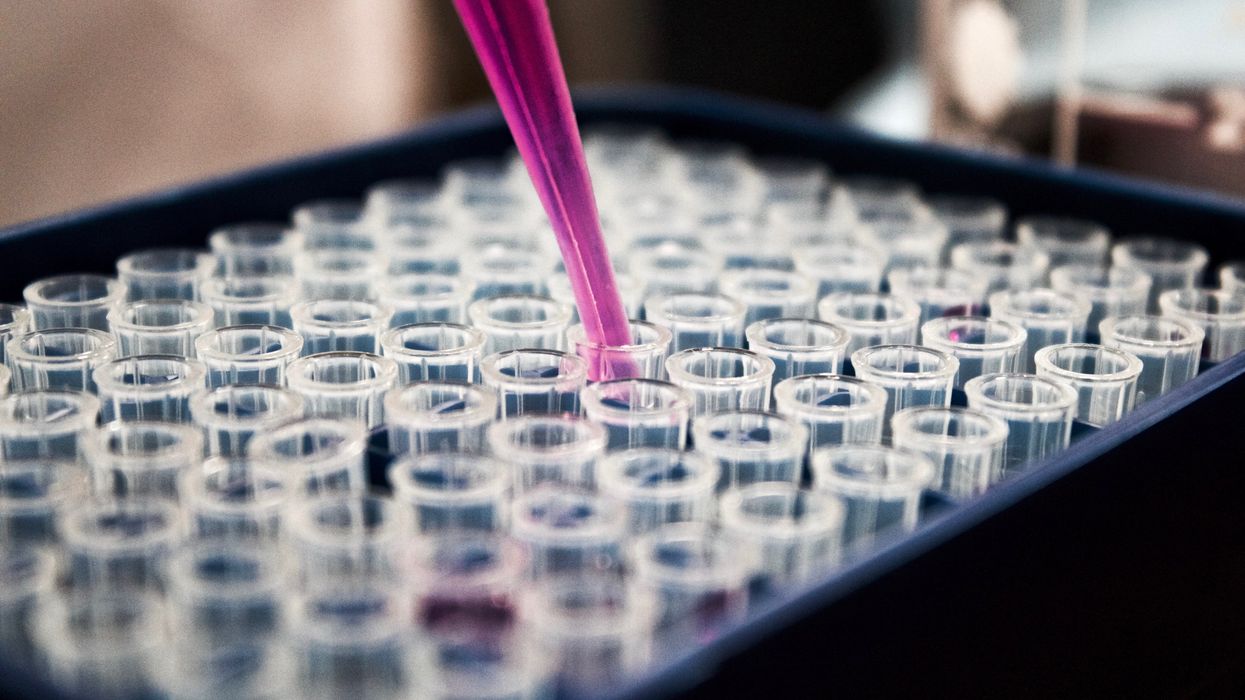
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
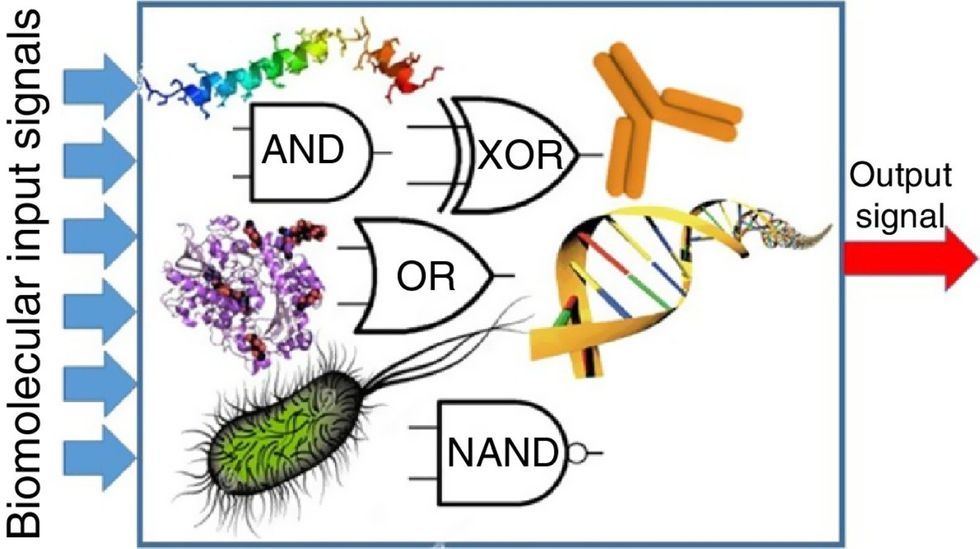
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.