Why Haven’t Researchers Developed an HIV Vaccine or Cure Yet?
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
A blood test for analysis of HIV.
Last week, top experts on HIV/AIDS convened in Amsterdam for the 22nd International AIDS conference, and the mood was not great. Even though remarkable advances in treating HIV have led to effective management for many people living with the disease, and its overall incidence has declined, there are signs that the virus could make a troubling comeback.
"In a perfect world, we'd get a vaccine like the HPV vaccine that was 100% effective and I think that's ultimately what we're going to strive for."
Growing resistance to current HIV drugs, a population boom in Sub-Saharan Africa, and insufficient public health resources are all poised to contribute to a second AIDS pandemic, according to published reports.
Already, the virus is nowhere near under control. Though the infection rate has declined 47 percent since its peak in 1996, last year 1.8 million people became newly infected with HIV around the world, and 37 million people are currently living with it. About 1 million people die of AIDS every year, making it the fourth biggest killer in low-income countries.
Leapsmag Editor-in-Chief Kira Peikoff reached out to Dr. Carl Dieffenbach, Director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, to find out what the U.S. government is doing to develop an HIV vaccine and cure. This interview has been edited and condensed for clarity.
What is the general trajectory of research in HIV/AIDS today?
We can break it down to two specific domains: focus on treatment and cure, and prevention.
Let's start with people living with HIV. This is the area where we've had the most success over the past 30 plus years, because we've taken a disease that was essentially a death sentence and converted it through the development of medications to a treatable chronic disease.
The second half of this equation is, can we cure or create a functional cure for people living with HIV? And the definition of functional cure would be the absence of circulating virus in the body in the absence of therapy. Essentially the human body would control the HIV infection within the individual. That is a much more, very early research stage of discovery. There are some interesting signals but it's still in need of innovation.
I'd like to make a contrast between what we are able to do with a virus called Hepatitis C and what we can do with the virus HIV. Hep C, with 12 weeks of highly active antiviral therapy, we can cure 95 to 100% of infections. With HIV, we cannot do that. The difference is the behavior of the virus. HIV integrates into the host's genome. Hep C is an RNA virus that stays in the cytoplasm of the cell and never gets into the DNA.
On the prevention side, we have two strategies: The first is pre-exposure prophylaxis. Then of course, we have the need for a safe, effective and durable HIV vaccine, which is a very active area of discovery. We've had some spectacular success with RV144, and we're following up on that success, and other vaccines are in the pipeline. Whether they are sufficient to provide the level of durability and activity is not yet clear, but progress has been made and there's still the need for innovation.
The most important breakthrough in the past 5 to 10 years has been the discovery of broad neutralizing monoclonal antibodies. They are proteins that the body makes, and not everybody who's HIV infected makes these antibodies, but we've been able to clone out these antibodies from certain individuals that are highly potent, and when used either singly or in combination, can truly neutralize the vast majority of HIV strains. Can those be used by themselves as treatment or as prevention? That is the question.
Can you explain more about RV144 and why you consider it a success?
Prior to RV144, we had run a number of vaccine studies and nothing had ever statistically shown to be protective. RV144 showed a level of efficacy of about 31 percent, which was statistically significant. Not enough to take forward into other studies, but it allowed us to generate some ideas about why this worked, go back to the drawing board, and redesign the immunogens to optimize and test the next generation for this vaccine. We just recently opened that new study, the follow-up to RV144, called HVTN702. That's up and enrolling and moving along quite nicely.

Carl Dieffenbach, Director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases
(Courtesy)
Where is that enrolling?
Primarily in Sub-Saharan Africa and South Africa.
When will you expect to see signals from that?
Between 2020 and 2021. It's complicated because the signal also takes into account the durability. After a certain time of vaccination, we're going to count up endpoints.
How would you explain the main scientific obstacle in the way of creating a very efficacious HIV vaccine?
Simply put, it's the black box of the human immune system. HIV employs a shield technology, and the virus is constantly changing its shield to protect itself, but there are some key parts of the virus that it cannot shield, so that's the trick – to be able to target that.
So, you're trying to find the Achilles' Heel of the virus?
Exactly. To make a flu vaccine or a Zika vaccine or even an Ebola vaccine, the virus is a little bit more forthcoming with the target. In HIV, the virus does everything in its power to hide the target, so we're dealing with a well-adapted [adversary] that actively avoids neutralization. That's the scientific challenge we face.
What's next?
On the vaccine side, we are currently performing, in collaboration with partners, two vaccine trials – HVTN702, which we talked about, and another one called 705. If either of those are highly successful, they would both require an additional phase 3 clinical trial before they could be licensed. This is an important but not final step. Then we would move into scale up to global vaccination. Those conversations have begun but they are not very far along and need additional attention.
What percent of people in the current trials would need to be protected to move on to phase 3?
Between 50 and 60 percent. That comes with this question of durability: how long does the vaccine last?
It also includes, can we simplify the vaccine regimen? The vaccines we're testing right now are multiple shots over a period of time. Can we get more like the polio or smallpox vaccine, a shot with a booster down the road?
We're dealing with sovereign nations. We're doing this in partnership, not as helicopter-type researchers.
If these current trials pan out, do you think kids in the developed world will end up getting an HIV vaccine one day? Or just people in-at risk areas?
That's a good question. I don't have an answer to that. In a perfect world, we'd get a vaccine like the HPV vaccine that was 100% effective and I think that's ultimately what we're going to strive for. That's where that second or third generation of vaccines that trigger broad neutralizing antibodies come in.
With any luck at all, globally, the combination of antiretroviral treatment, pre-exposure prophylaxis and other prevention and treatment strategies will lower the incidence rate where the HIV pandemic continues to wane, and we will then be able to either target the vaccine or roll it out in a way that is both cost effective and destigmatizing.
And also, what does the country want? We're dealing with sovereign nations. We're doing this in partnership, not as helicopter-type researchers.
How close do you think we are globally to eradicating HIV infections?
Eradication's a big word. It means no new infections. We are nowhere close to eradicating HIV. Whether or not we can continue to bend the curve on the epidemic and have less infections so that the total number of people continues to decline over time, I think we can achieve that if we had the political will. And that's not just the U.S. political will. That's the will of the world. We have the tools, albeit they're not perfect. But that's where a vaccine that is efficacious and simple to deliver could be the gamechanger.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Kirstie Ennis, an Afghanistan veteran who survived a helicopter crash but lost a limb, pictured in May 2021 at Two Rivers Park in Colorado.
In June 2012, Kirstie Ennis was six months into her second deployment to Afghanistan and recently promoted to sergeant. The helicopter gunner and seven others were three hours into a routine mission of combat resupplies and troop transport when their CH-53D helicopter went down hard.
Miraculously, all eight people onboard survived, but Ennis' injuries were many and severe. She had a torn rotator cuff, torn labrum, crushed cervical discs, facial fractures, deep lacerations and traumatic brain injury. Despite a severely fractured ankle, doctors managed to save her foot, for a while at least.
In November 2015, after three years of constant pain and too many surgeries to count, Ennis relented. She elected to undergo a lower leg amputation but only after she completed the 1,000-mile, 72-day Walking with the Wounded journey across the UK.
On Veteran's Day of that year, on the other side of the country, orthopedic surgeon Cato Laurencin announced a moonshot challenge he was setting out to achieve on behalf of wounded warriors like Ennis: the Hartford Engineering A Limb (HEAL) Project.
Laurencin, who is a University of Connecticut professor of chemical, materials and biomedical engineering, teamed up with experts in tissue bioengineering and regenerative medicine from Harvard, Columbia, UC Irvine and SASTRA University in India. Laurencin and his colleagues at the Connecticut Convergence Institute for Translation in Regenerative Engineering made a bold commitment to regenerate an entire limb within 15 years – by the year 2030.

Dr. Cato Laurencin pictured in his office at UConn.
Photo Credit: UConn
Regenerative Engineering -- A Whole New Field
Limb regeneration in humans has been a medical and scientific fascination for decades, with little to show for the effort. However, Laurencin believes that if we are to reach the next level of 21st century medical advances, this puzzle must be solved.
An estimated 185,000 people undergo upper or lower limb amputation every year. Despite the significant advances in electromechanical prosthetics, these individuals still lack the ability to perform complex functions such as sensation for tactile input, normal gait and movement feedback. As far as Laurencin is concerned, the only clinical answer that makes sense is to regenerate a whole functional limb.
Laurencin feels other regeneration efforts were hampered by their siloed research methods with chemists, surgeons, engineers all working separately. Success, he argues, requires a paradigm shift to a trans-disciplinary approach that brings together cutting-edge technologies from disparate fields such as biology, material sciences, physical, chemical and engineering sciences.
As the only surgeon ever inducted into the academies of Science, Medicine and Innovation, Laurencin is uniquely suited for the challenge. He is regarded as the founder of Regenerative Engineering, defined as the convergence of advanced materials sciences, stem cell sciences, physics, developmental biology and clinical translation for the regeneration of complex tissues and organ systems.
But none of this is achievable without early clinician participation across scientific fields to develop new technologies and a deeper understanding of how to harness the body's innate regenerative capabilities. "When I perform a surgical procedure or something is torn or needs to be repaired, I count on the body being involved in regenerating tissue," he says. "So, understanding how the body works to regenerate itself and harnessing that ability is an important factor for the regeneration process."
The Birth of the Vision
Laurencin's passion for regeneration began when he was a sports medicine fellow at Cornell University Medical Center in the early 1990s. There he saw a significant number of injuries to the anterior cruciate ligament (ACL), the major ligament that stabilizes the knee. He believed he could develop a better way to address those injuries using biomaterials to regenerate the ligament. He sketched out a preliminary drawing on a napkin one night over dinner. He has spent the next 30 years regenerating tissues, including the patented L-C ligament.
As chair of Orthopaedic Surgery at the University of Virginia during the peak of the wars in Iraq and Afghanistan, Laurencin treated military personnel who survived because of improved helmets, body armor and battlefield medicine but were left with more devastating injuries, including traumatic brain injuries and limb loss.
"I was so honored to care for them and I so admired their steadfast courage that I became determined to do something big for them," says Laurencin.
When he tells people about his plans to regrow a limb, he gets a lot of eye rolls, which he finds amusing but not discouraging. Growing bone cells was relatively new when he was first focused on regenerating bone in 1987 at MIT; in 2007 he was well on his way to regenerating ligaments at UVA when many still doubted that ligaments could even be reconstructed. He and his team have already regenerated torn rotator cuff tendons and ACL ligaments using a nano-textured fabric seeded with stem cells.
Even as a finalist for the $4 million NIH Pioneer Award for high-risk/high-reward research, he faced a skeptical scientific audience in 2014. "They said, 'Well what do you plan to do?' I said 'I plan to regenerate a whole limb in people.' There was a lot of incredulousness. They stared at me and asked a lot of questions. About three days later, I received probably the best score I've ever gotten on an NIH grant."
In the Thick of the Science
Humans are born with regenerative abilities--two-year-olds have regrown fingertips--but lose that ability with age. Salamanders are the only vertebrates that can regenerate lost body parts as adults; axolotl, the rare Mexican salamander, can grow extra limbs.
The axolotl is important as a model organism because it is a four-footed vertebrate with a similar body plan to humans. Mapping the axolotl genome in 2018 enhanced scientists' genetic understanding of their evolution, development, and regeneration. Being easy to breed in captivity allowed the HEAL team to closely study these amphibians and discover a new cell type they believe may shed light on how to mimic the process in humans.
"Whenever limb regeneration takes place in the salamander, there is a huge amount of something called heparan sulfate around that area," explains Laurencin. "We thought, 'What if this heparan sulfate is the key ingredient to allowing regeneration to take place?' We found these groups of cells that were interspersed in tissues during the time of regeneration that seemed to have connections to each other that expressed this heparan sulfate."
Called GRID (Groups that are Regenerative, Interspersed and Dendritic), these cells were also recently discovered in mice. While GRID cells don't regenerate as well in mice as in salamanders, finding them in mammals was significant.
"If they're found in mice. we might be able to find these in humans in some form," Laurencin says. "We think maybe it will help us figure out regeneration or we can create cells that mimic what grid cells do and create an artificial grid cell."
What Comes Next?
Laurencin and his team have individually engineered and made every single tissue in the lower limb, including bone, cartilage, ligament, skin, nerve, blood vessels. Regenerating joints and joint tissue is the next big mile marker, which Laurencin sees as essential to regenerating a limb that functions and performs in the way he envisions.
"Using stem cells and amnion tissue, we can regenerate joints that are damaged, and have severe arthritis," he says. "We're making progress on all fronts, and making discoveries we believe are going to be helping people along the way."
That focus and advancement is vital to Ennis. After laboring over the decision to have her leg amputated below the knee, she contracted MRSA two weeks post-surgery. In less than a month, she went from a below-the-knee-amputee to a through-the-knee amputee to an above-the-knee amputee.
"A below-the-knee amputation is night-and-day from above-the-knee," she said. "You have to relearn everything. You're basically a toddler."
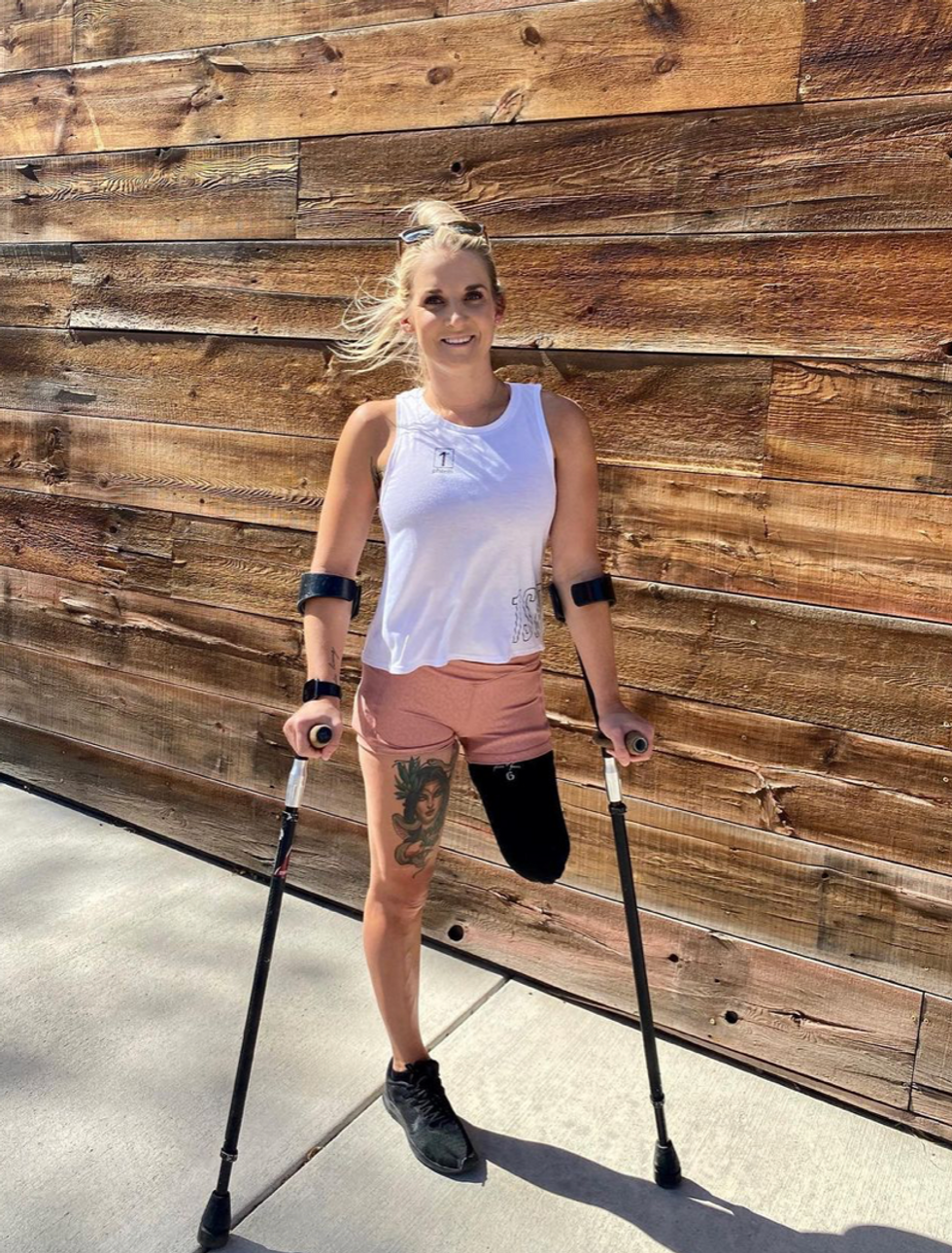
Kirstie Ennis pictured in July 2020.
Photo Credit: Ennis' Instagram
The clock is ticking on the timeline Laurencin set for himself. Nine years might seem like forever if you're doing time but it might appear fleeting when you're trying to create something that's never been done before. But Laurencin isn't worried. He's convinced time is on his side.
"Every week, I receive an email or a call from someone, maybe a mother whose child has lost a finger or I'm in communication with a disabled American veteran who wants to know how the progress is going. That energizes me to continue to work hard to try to create these sorts of solutions because we're talking about people and their lives."
He devotes about 60 hours a week to the project and the roughly 100 students, faculty and staff who make up the HEAL team at the Convergence Institute seem acutely aware of what's at stake and appear equally dedicated.
"We're in the thick of the science in terms of making this happen," says Laurencin. "We've moved from making the impossible possible to making the possible a reality. That's what science is all about."
7 Reasons Why We Should Not Need Boosters for COVID-19
A top infectious disease physician explains why immunity derived from natural infection and the vaccines should be long-lasting.
There are at least 7 reasons why immunity after vaccination or infection with COVID-19 should likely be long-lived. If durable, I do not think boosters will be necessary in the future, despite CEOs of pharmaceutical companies (who stand to profit from boosters) messaging that they may and readying such boosters. To explain these reasons, let's orient ourselves to the main components of the immune system.
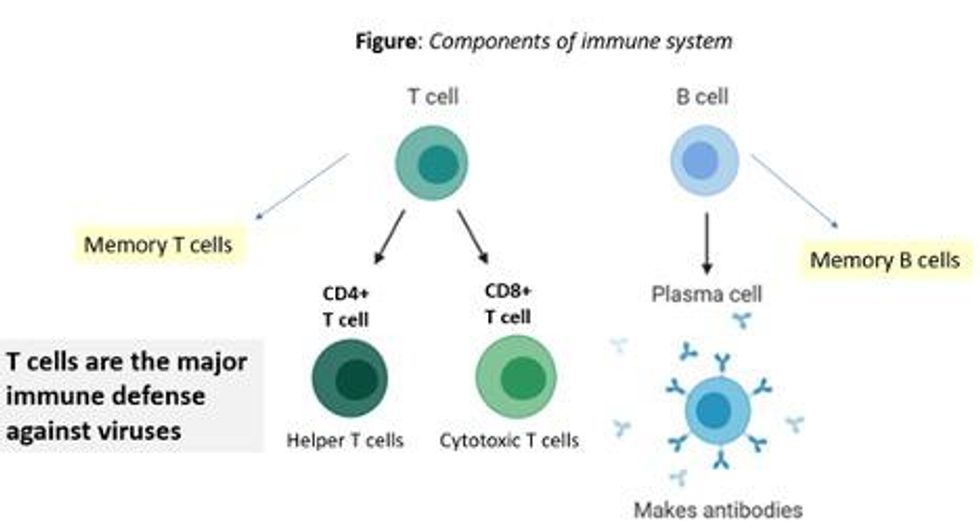
There are two major arms of the immune system: B cells (which produce antibodies) and T cells (which are formed specifically to attack and kill pathogens). T cells are divided into two types, CD4 cells ("helper" T cells) and CD8 cells ("cytotoxic" T cells).
Each arm, once stimulated by infection or vaccine, should hopefully make "memory" banks. So if the body sees the pathogen in the future, these defenses should come roaring back to attack the virus and protect you from getting sick. Plenty of research in COVID-19 indicates a likely long-lasting response to the vaccine or infection. Here are seven of the most compelling reasons:
REASON 1: Memory B Cells Are Produced By Vaccines and Natural Infection
In one study, 12 volunteers who had never had Covid-19--and were fully vaccinated with two Pfizer/BioNTech shots-- underwent biopsies of their lymph nodes. This is where memory B cells are stored in places called "germinal centers". The biopsies were performed three, four, six, and seven weeks after the first mRNA vaccine shot, and were stained to reveal that germinal center memory B cells in the lymph nodes increased in concentration over time.
Natural infection also generates memory B cells. Even after antibody levels wane over time, strong memory B cells were detected in the blood of individuals six and eight months after infection in different studies. Indeed, the half-lives of the memory B cells seen in the study examining patients 8 months after COVID-19 led the authors to conclude that "B cell memory to SARS-CoV-2 was robust and is likely long-lasting." Reason #2 tells us that memory B cells can be active for a very long time indeed.
REASON #2: Memory B Cells Can Produce Neutralizing Antibodies If They See Infection Again Decades Later
Demonstrated production of memory B cells after vaccination or natural infection with COVID-19 is so important because memory B cells, once generated, can be activated to produce high levels of neutralizing antibodies against the pathogen even if encountered many years after the initial exposure. In one amazing study (published in 2008), researchers isolated memory B cells against the 1918 flu strain from the blood of 32 individuals aged 91-101 years. These people had been born on or before 1915 and had survived that pandemic.
Their memory B cells, when exposed to the 1918 flu strain in a test tube, generated high levels of neutralizing antibodies against the virus -- antibodies that then protected mice from lethal infection with this deadly strain. The ability of memory B cells to produce complex antibody responses against an infection nine decades after exposure speaks to their durability.
REASON #3: Vaccines or Natural Infection Trigger Strong Memory T Cell Immunity
All of the trials of the major COVID-19 vaccine candidates measured strong T cell immunity following vaccination, most often assessed by measuring SARS-CoV-2 specific T cells in the phase I/II safety and immunogenicity studies. There are a number of studies that demonstrate the production of strong T cell immunity to COVID-19 after natural infection as well, even when the infection was mild or asymptomatic.
The same study that showed us robust memory B cell production 8 months after natural infection also demonstrated strong and sustained memory T cell production. In fact, the half-lives of the memory T cells in this cohort were long (~125-225 days for CD8+ and ~94-153 days for CD4+ T cells), comparable to the 123-day half-life observed for memory CD8+ T cells after yellow fever immunization (a vaccine usually given once over a lifetime).
A recent study of individuals recovered from COVID-19 show that the initial T cells generated by natural infection mature and differentiate over time into memory T cells that will be "put in the bank" for sustained periods.
REASON #4: T Cell Immunity Following Vaccinations for Other Infections Is Long-Lasting
Last year, we were fortunate to be able to measure how T cell immunity is generated by COVID-19 vaccines, which was not possible in earlier eras when vaccine trials were done for other infections (such as measles, mumps, rubella, pertussis, diphtheria). Antibodies are just the "tip of the iceberg" when assessing the response to vaccination, but were the only arm of the immune response that could be measured following vaccination in the past.
Measuring pathogen-specific T cell responses takes sophisticated technology. However, T cell responses, when assessed years after vaccination for other pathogens, has been shown to be long-lasting. For example, in one study of 56 volunteers who had undergone measles vaccination when they were much younger, strong CD8 and CD4 cell responses to vaccination could be detected up to 34 years later.
REASON #5: T Cell Immunity to Related Coronaviruses That Caused Severe Disease is Long-Lasting
SARS-CoV-2 is a coronavirus that causes severe disease, unlike coronaviruses that cause the common cold. Two other coronaviruses in the recent past caused severe disease, specifically Severely Acute Respiratory Distress Syndrome (SARS) in late 2002-2003 and Middle East Respiratory Syndrome (MERS) in 2011.
A study performed in 2020 demonstrated that the blood of 23 recovered SARS patients possess long-lasting memory T cells that were still reactive to SARS 17 years after the outbreak in 2003. Many scientists expect that T cell immunity to SARS-CoV-2 will be equally durable to that of its cousin.
REASON #6: T Cell Responses from Vaccination and Natural Infection With the Ancestral Strain of COVID-19 Are Robust Against Variants
Even though antibody responses from vaccination may be slightly lower against various COVID-19 variants of concern that have emerged in recent months, T cell immunity after vaccination has been shown to be unperturbed by mutations in the spike protein (in the variants). For instance, T cell responses after mRNA vaccines maintained strong activity against different variants (including P.1 Brazil variant, B.1.1.7 UK variant, B.1.351 South Africa variant and the CA.20.C California variant) in a recent study.
Another study showed that the vaccines generated robust T cell immunity that was unfazed by different variants, including B.1.351 and B.1.1.7. The CD4 and CD8 responses generated after natural infection are equally robust, showing activity against multiple "epitopes" (little segments) of the spike protein of the virus. For instance, CD8 cells responds to 52 epitopes and CD4 cells respond to 57 epitopes across the spike protein, so that a few mutations in the variants cannot knock out such a robust and in-breadth T cell response. Indeed, a recent paper showed that mRNA vaccines were 97.4 percent effective against severe COVID-19 disease in Qatar, even when the majority of circulating virus there was from variants of concern (B.1.351 and B.1.1.7).
REASON #7: Coronaviruses Don't Mutate Quickly Like Influenza, Which Requires Annual Booster Shots
Coronaviruses are RNA viruses, like influenza and HIV (which is actually a retrovirus), but do not mutate as quickly as either one. The reason that coronaviruses don't mutate very rapidly is that their replicating mechanism (polymerase) has a strong proofreading mechanism: If the virus mutates, it usually goes back and self-corrects. Mutations can arise with high rates of replication when transmission is very frequent -- as has been seen in recent months with the emergence of SARS-CoV-2 variants during surges. However, the COVID-19 virus will not be mutating like this when we tamp down transmission with mass vaccination.
In conclusion, I and many of my infectious disease colleagues expect the immunity from natural infection or vaccination to COVID-19 to be durable. Let's put discussion of boosters aside and work hard on global vaccine equity and distribution since the pandemic is not over until it is over for us all.

