Your Community and COVID-19: How to Make Sense of the Numbers Where You Live
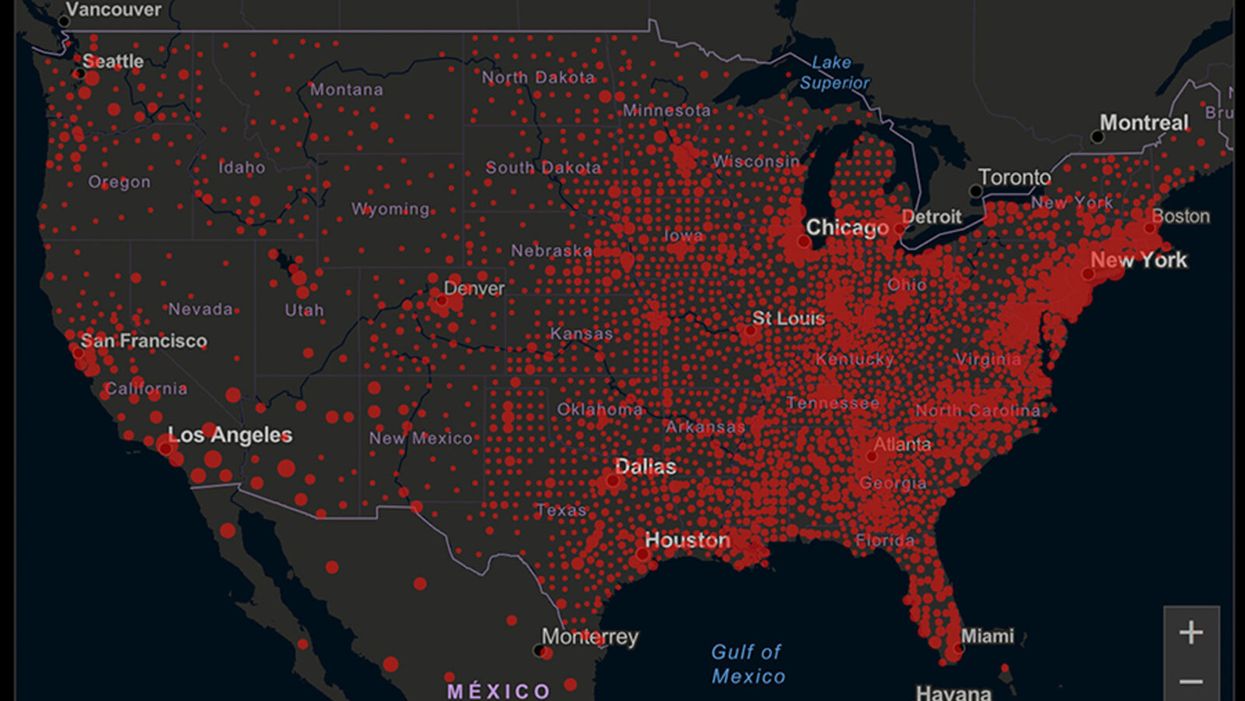
A map of cumulative known cases of COVID-19 in the U.S., as of June 12th, 2020.
Have you felt a bit like an armchair epidemiologist lately? Maybe you've been poring over coronavirus statistics on your county health department's website or on the pages of your local newspaper.
If the percentage of positive tests steadily stays under 8 percent, that's generally a good sign.
You're likely to find numbers and charts but little guidance about how to interpret them, let alone use them to make day-to-day decisions about pandemic safety precautions.
Enter the gurus. We asked several experts to provide guidance for laypeople about how to navigate the numbers. Here's a look at several common COVID-19 statistics along with tips about how to understand them.
Case Counts: Consider the Context
The number of confirmed COVID-19 cases in American counties is widely available. Local and state health departments should provide them online, or you can easily look them up at The New York Times' coronavirus database. However, you need to be cautious about interpreting them.
"Case counts are the obvious numbers to look at. But they're probably the hardest thing to sort out," said Dr. Jeff Martin, an epidemiologist at the University of California at San Francisco.
That's because case counts by themselves aren't a good window into how the coronavirus is affecting your community since they rely on testing. And testing itself varies widely from day to day and community to community.
"The more testing that's done, the more infections you'll pick up," explained Dr. F. Perry Wilson, a physician at Yale University. The numbers can also be thrown off when tests are limited to certain groups of people.
"If the tests are being mostly given to people with a high probability of having been infected -- for example, they have had symptoms or work in a high-risk setting -- then we expect lots of the tests to be positive. But that doesn't tell us what proportion of the general public is likely to have been infected," said Eleanor Murray, an epidemiologist at Boston University.
These Stats Are More Meaningful
According to Dr. Wilson, it's more useful to keep two other statistics in mind: the number of COVID tests that are being performed in your community and the percentage that turn up positive, showing that people have the disease. (These numbers may or may not be available locally. Check the websites of your community's health department and local news media outlets.)
If the number of people being tested is going up, but the percentage of positive tests is going down, Dr. Wilson said, that's a good sign. But if both numbers are going up – the number of people tested and the percentage of positive results – then "that's a sign that there are more infections burning in the community."
It's especially worrisome if the percentage of positive cases is growing compared to previous days or weeks, he said. According to him, that's a warning of a "high-risk situation."
Dr. George Rutherford, an epidemiologist at University of California at San Francisco, offered this tip: If the percentage of positive tests steadily stays under 8 percent, that's generally a good sign.
There's one more caveat about case counts. It takes an average of a week for someone to be infected with COVID-19, develop symptoms, and get tested, Dr. Rutherford said. It can take an additional several days for those test results to be reported to the county health department. This means that case numbers don't represent infections happening right now, but instead are a picture of the state of the pandemic more than a week ago.
Hospitalizations: Focus on Current Statistics
You should be able to find numbers about how many people in your community are currently hospitalized – or have been hospitalized – with diagnoses of COVID-19. But experts say these numbers aren't especially revealing unless you're able to see the number of new hospitalizations over time and track whether they're rising or falling. This number often isn't publicly available, however.
If new hospitalizations are increasing, "you may want to react by being more careful yourself."
And there's an important caveat: "The problem with hospitalizations is that they do lag," UC San Francisco's Dr. Martin said, since it takes time for someone to become ill enough to need to be hospitalized. "They tell you how much virus was being transmitted in your community 2 or 2.5 weeks ago."
Also, he said, people should be cautious about comparing new hospitalization rates between communities unless they're adjusted to account for the number of more-vulnerable older people.
Still, if new hospitalizations are increasing, he said, "you may want to react by being more careful yourself."
Deaths: They're an Even More Delayed Headline
Cable news networks obsessively track the number of coronavirus deaths nationwide, and death counts for every county in the country are available online. Local health departments and media websites may provide charts tracking the growth in deaths over time in your community.
But while death rates offer insight into the disease's horrific toll, they're not useful as an instant snapshot of the pandemic in your community because severely ill patients are typically sick for weeks. Instead, think of them as a delayed headline.
"These numbers don't tell you what's happening today. They tell you how much virus was being transmitted 3-4 weeks ago," Dr. Martin said.
'Reproduction Value': It May Be Revealing
You're not likely to find an available "reproduction value" for your community, but it is available for your state and may be useful.
A reproduction value, also known as R0 or R-naught, "tells us how many people on average we expect will be infected from a single case if we don't take any measures to intervene and if no one has been infected before," said Boston University's Murray.
As The New York Times explained, "R0 is messier than it might look. It is built on hard science, forensic investigation, complex mathematical models — and often a good deal of guesswork. It can vary radically from place to place and day to day, pushed up or down by local conditions and human behavior."
It may be impossible to find the R0 for your community. However, a website created by data specialists is providing updated estimates of a related number -- effective reproduction number, or Rt – for each state. (The R0 refers to how infectious the disease is in general and if precautions aren't taken. The Rt measures its infectiousness at a specific time – the "t" in Rt.) The site is at rt.live.
"The main thing to look at is whether the number is bigger than 1, meaning the outbreak is currently growing in your area, or smaller than 1, meaning the outbreak is currently decreasing in your area," Murray said. "It's also important to remember that this number depends on the prevention measures your community is taking. If the Rt is estimated to be 0.9 in your area and you are currently under lockdown, then to keep it below 1 you may need to remain under lockdown. Relaxing the lockdown could mean that Rt increases above 1 again."
"Whether they're on the upswing or downswing, no state is safe enough to ignore the precautions about mask wearing and social distancing."
Keep in mind that you can still become infected even if an outbreak in your community appears to be slowing. Low risk doesn't mean no risk.
Putting It All Together: Why the Numbers Matter
So you've reviewed COVID-19 statistics in your community. Now what?
Dr. Wilson suggests using the data to remind yourself that the coronavirus pandemic "is still out there. You need to take it seriously and continue precautions," he said. "Whether they're on the upswing or downswing, no state is safe enough to ignore the precautions about mask wearing and social distancing. 'My state is doing well, no one I know is sick, is it time to have a dinner party?' No."
He also recommends that laypeople avoid tracking COVID-19 statistics every day. "Check in once a week or twice a month to see how things are going," he suggested. "Don't stress too much. Just let it remind you to put that mask on before you get out of your car [and are around others]."
Leading XPRIZE Healthspan and Beating Negativity with Dr. Peter Diamandis
XPRIZE founder and chairman Peter Diamandis launches XPRIZE Healthspan at an event on November 29.
A new competition by the XPRIZE Foundation is offering $101 million to researchers who discover therapies that give a boost to people aged 65-80 so their bodies perform more like when they were middle-aged.
For today’s podcast episode, I talked with Dr. Peter Diamandis, XPRIZE’s founder and executive chairman. Under Peter’s leadership, XPRIZE has launched 27 previous competitions with over $300 million in prize purses. The latest contest aims to enhance healthspan, or the period of life when older people can play with their grandkids without any restriction, disability or disease. Such breakthroughs could help prevent chronic diseases that are closely linked to aging. These illnesses are costly to manage and threaten to overwhelm the healthcare system, as the number of Americans over age 65 is rising fast.
In this competition, called XPRIZE Healthspan, multiple awards are available, depending on what’s achieved, with support from the nonprofit Hevolution Foundation and Chip Wilson, the founder of Lululemon and nonprofit SOLVE FSHD. The biggest prize, $81 million, is for improvements in cognition, muscle and immunity by 20 years. An improvement of 15 years will net $71 million, and 10 years will net $61 million.
In our conversation for this episode, Peter talks about his plans for XPRIZE Healthspan and why exponential technologies make the current era - even with all of its challenges - the most exciting time in human history. We discuss the best mental outlook that supports a person in becoming truly innovative, as well as the downsides of too much risk aversion. We talk about how to overcome the negativity bias in ourselves and in mainstream media, how Peter has shifted his own mindset to become more positive over the years, how to inspire a culture of innovation, Peter’s personal recommendations for lifestyle strategies to live longer and healthier, the innovations we can expect in various fields by 2030, the future of education and the importance of democratizing tech and innovation.
In addition to Peter’s pioneering leadership of XPRIZE, he is also the Executive Founder of Singularity University. In 2014, he was named by Fortune as one of the “World’s 50 Greatest Leaders.” As an entrepreneur, he’s started over 25 companies in the areas of health-tech, space, venture capital and education. He’s Co-founder and Vice-Chairman of two public companies, Celularity and Vaxxinity, plus being Co-founder & Chairman of Fountain Life, a fully-integrated platform delivering predictive, preventative, personalized and data-driven health. He also serves as Co-founder of BOLD Capital Partners, a venture fund with a half-billion dollars under management being invested in exponential technologies and longevity companies. Peter is a New York Times Bestselling author of four books, noted during our conversation and in the show notes of this episode. He has degrees in molecular genetics and aerospace engineering from MIT and holds an M.D. from Harvard Medical School.
Show links
- Peter Diamandis bio
- New XPRIZE Healthspan
- Peter Diamandis books
- 27 XPRIZE competitions and counting
- Life Force by Peter Diamandis and Tony Robbins
- Peter Diamandis Twitter
- Longevity Insider newsletter – AI identifies the news
- Peter Diamandis Longevity Handbook
- Hevolution funding for longevity

XPRIZE Founder Peter Diamandis speaks with Mehmoud Khan, CEO of Hevolution Foundation, at the launch of XPRIZE Healthspan.
Hevolution Foundation
Important findings are starting to emerge from research on how genes shape the human response to the Covid virus.
From infections with no symptoms to why men are more likely to be hospitalized in the ICU and die of COVID-19, new research shows that your genes play a significant role
Early in the pandemic, genetic research focused on the virus because it was readily available. Plus, the virus contains only 30,000 bases in a dozen functional genes, so it's relatively easy and affordable to sequence. Additionally, the rapid mutation of the virus and its ability to escape antibody control fueled waves of different variants and provided a reason to follow viral genetics.
In comparison, there are many more genes of the human immune system and cellular functions that affect viral replication, with about 3.2 billion base pairs. Human studies require samples from large numbers of people, the analysis of each sample is vastly more complex, and sophisticated computer analysis often is required to make sense of the raw data. All of this takes time and large amounts of money, but important findings are beginning to emerge.
Asymptomatics
About half the people exposed to SARS-CoV-2, the virus that causes the COVID-19 disease, never develop symptoms of this disease, or their symptoms are so mild they often go unnoticed. One piece of understanding the phenomena came when researchers showed that exposure to OC43, a common coronavirus that results in symptoms of a cold, generates immune system T cells that also help protect against SARS-CoV-2.
Jill Hollenbach, an immunologist at the University of California at San Francisco, sought to identify the gene behind that immune protection. Most COVID-19 genetic studies are done with the most seriously ill patients because they are hospitalized and thus available. “But 99 percent of people who get it will never see the inside of a hospital for COVID-19,” she says. “They are home, they are not interacting with the health care system.”
Early in the pandemic, when most labs were shut down, she tapped into the National Bone Marrow Donor Program database. It contains detailed information on donor human leukocyte antigens (HLAs), key genes in the immune system that must match up between donor and recipient for successful transplants of marrow or organs. Each HLA can contain alleles, slight molecular differences in the DNA of the HLA, which can affect its function. Potential HLA combinations can number in the tens of thousands across the world, says Hollenbach, but each person has a smaller number of those possible variants.
She teamed up with the COVID-19 Citizen Science Study a smartphone-based study to track COVID-19 symptoms and outcomes, to ask persons in the bone marrow donor registry about COVID-19. The study enlisted more than 30,000 volunteers. Those volunteers already had their HLAs annotated by the registry, and 1,428 tested positive for the virus.
Analyzing five key HLAs, she found an allele in the gene HLA-B*15:01 that was significantly overrepresented in people who didn’t have any symptoms. The effect was even stronger if a person had inherited the allele from both parents; these persons were “more than eight times more likely to remain asymptomatic than persons who did not carry the genetic variant,” she says. Altogether this HLA was present in about 10 percent of the general European population but double that percentage in the asymptomatic group. Hollenbach and her colleagues were able confirm this in other different groups of patients.
What made the allele so potent against SARS-CoV-2? Part of the answer came from x-ray crystallography. A key element was the molecular shape of parts of the cold virus OC43 and SARS-CoV-2. They were virtually identical, and the allele could bind very tightly to them, present their molecular antigens to T cells, and generate an extremely potent T cell response to the viruses. And “for whatever reasons that generated a lot of memory T cells that are going to stick around for a long time,” says Hollenbach. “This T cell response is very early in infection and ramps up very quickly, even before the antibody response.”
Understanding the genetics of the immune response to SARS-CoV-2 is important because it provides clues into the conditions of T cells and antigens that support a response without any symptoms, she says. “It gives us an opportunity to think about whether this might be a vaccine design strategy.”
Dead men
A researcher at the Leibniz Institute of Virology in Hamburg Germany, Guelsah Gabriel, was drawn to a question at the other end of the COVID-19 spectrum: why men more likely to be hospitalized and die from the infection. It wasn't that men were any more likely to be exposed to the virus but more likely, how their immune system reacted to it
Several studies had noted that testosterone levels were significantly lower in men hospitalized with COVID-19. And, in general, the lower the testosterone, the worse the prognosis. A year after recovery, about 30 percent of men still had lower than normal levels of testosterone, a condition known as hypogonadism. Most of the men also had elevated levels of estradiol, a female hormone (https://pubmed.ncbi.nlm.nih.gov/34402750/).
Every cell has a sex, expressing receptors for male and female hormones on their surface. Hormones docking with these receptors affect the cells' internal function and the signals they send to other cells. The number and role of these receptors varies from tissue to tissue.
Gabriel began her search by examining whole exome sequences, the protein-coding part of the genome, for key enzymes involved in the metabolism of sex hormones. The research team quickly zeroed in on CYP19A1, an enzyme that converts testosterone to estradiol. The gene that produces this enzyme has a number of different alleles, the molecular variants that affect the enzyme's rate of metabolizing the sex hormones. One genetic variant, CYP19A1 (Thr201Met), is typically found in 6.2 percent of all people, both men and women, but remarkably, they found it in 68.7 percent of men who were hospitalized with COVID-19.
Lung surprise
Lungs are the tissue most affected in COVID-19 disease. Gabriel wondered if the virus might be affecting expression of their target gene in the lung so that it produces more of the enzyme that converts testosterone to estradiol. Studying cells in a petri dish, they saw no change in gene expression when they infected cells of lung tissue with influenza and the original SARS-CoV viruses that caused the SARS outbreak in 2002. But exposure to SARS-CoV-2, the virus responsible for COVID-19, increased gene expression up to 40-fold, Gabriel says.
Did the same thing happen in humans? Autopsy examination of patients in three different cites found that “CYP19A1 was abundantly expressed in the lungs of COVID-19 males but not those who died of other respiratory infections,” says Gabriel. This increased enzyme production led likely to higher levels of estradiol in the lungs of men, which “is highly inflammatory, damages the tissue, and can result in fibrosis or scarring that inhibits lung function and repair long after the virus itself has disappeared.” Somehow the virus had acquired the capacity to upregulate expression of CYP19A1.
Only two COVID-19 positive females showed increased expression of this gene. The menopause status of these women, or whether they were on hormone replacement therapy was not known. That could be important because female hormones have a protective effect for cardiovascular disease, which women often lose after going through menopause, especially if they don’t start hormone replacement therapy. That sex-specific protection might also extend to COVID-19 and merits further study.
The team was able to confirm their findings in golden hamsters, the animal model of choice for studying COVID-19. Testosterone levels in male animals dropped 5-fold three days after infection and began to recover as viral levels declined. CYP19A1 transcription increased up to 15-fold in the lungs of the male but not the females. The study authors wrote, “Virus replication in the male lungs was negatively associated with testosterone levels.”
The medical community studying COVID-19 has slowly come to recognize the importance of adipose tissue, or fat cells. They are known to express abundant levels of CYP19A1 and play a significant role as metabolic tissue in COVID-19. Gabriel adds, “One of the key findings of our study is that upon SARS-CoV-2 infection, the lung suddenly turns into a metabolic organ by highly expressing” CYP19A1.
She also found evidence that SARS-CoV-2 can infect the gonads of hamsters, thereby likely depressing circulating levels of sex hormones. The researchers did not have autopsy samples to confirm this in humans, but others have shown that the virus can replicate in those tissues.
A possible treatment
Back in the lab, substituting low and high doses of testosterone in SARS-COV-2 infected male hamsters had opposite effects depending on testosterone dosage used. Gabriel says that hormone levels can vary so much, depending on health status and age and even may change throughout the day, that “it probably is much better to inhibit the enzyme” produced by CYP19A1 than try to balance the hormones.
Results were better with letrozole, a drug approved to treat hypogonadism in males, which reduces estradiol levels. The drug also showed benefit in male hamsters in terms of less severe disease and faster recovery. She says more details need to be worked out in using letrozole to treat COVID-19, but they are talking with hospitals about clinical trials of the drug.
Gabriel has proposed a four hit explanation of how COVID-19 can be so deadly for men: the metabolic quartet. First is the genetic risk factor of CYP19A1 (Thr201Met), then comes SARS-CoV-2 infection that induces even greater expression of this gene and the deleterious increase of estradiol in the lung. Age-related hypogonadism and the heightened inflammation of obesity, known to affect CYP19A1 activity, are contributing factors in this deadly perfect storm of events.
Studying host genetics, says Gabriel, can reveal new mechanisms that yield promising avenues for further study. It’s also uniting different fields of science into a new, collaborative approach they’re calling “infection endocrinology,” she says.