Your Genetic Data Is The New Oil. These Startups Will Pay to Rent It.

A doctor conducts a test on DNA.
Perhaps you're one of the 12 million people who has spit into a tube in recent years to learn the secrets in your genetic code, like your ancestry, your health risks, or your carrier status for certain diseases. If you haven't participated in direct-to-consumer genetic testing, you may know someone who has.
It's for people who want more control over their genetic data--plus a share of the proceeds when and if that data is used.
Mountains of genomic data have been piling up steeply over the last several years, but according to some experts, not enough research and drug discovery is being done with the data collected, and customers rarely have a say in how their data is used. Now, a slew of ambitious startup companies are bringing together the best of blockchain technology and human genomics to help solve these problems.
But First, Why Is Your Genome So Valuable?
Access to genetic information is an obvious boon to scientific and medical progress. In the right hands, it has the potential to save lives and reduce suffering — by facilitating the development of better, safer, more targeted treatments and by shedding light on the role of genetics in countless diseases and medical conditions.
Research requiring access to direct-to-consumer (DTC) genomic data is already well underway. For example, 23andMe, the popular California-based DTC genetic testing company, has published 107 research articles so far, as of this May, using data from their five million-plus customers around the world. Their website states that, on average, of the 80 percent of their customers who have opted to share their genomic data for research purposes, each "individual contributes to 200 different research studies."
And this July, a new collaboration was announced between 23andMe and GlaxoSmithKline, the London-based pharmaceutical company. GlaxoSmithKline will be using data from 23andMe customers to develop new medical treatments, while 23andMe will receive $300 million from the four-year deal. Both companies are poised to profit significantly from their union.
Should 23andMe's customers share in the gains? Peter Pitts, president of the Center for Medicine in the Public Interest, believes they should. "Are they going to offer rebates to people who opt in, so their customers aren't paying for the privilege of 23andMe working with a for-profit company in a for-profit research project?" Pitts told NBC. So far, 23andMe has not announced any plans to share profits with their customers.
But outside of such major partnerships, many researchers are frustrated by the missed opportunities to dig deeper into the correlations between genetics and disease. That's because people's de-identified genomic information is "essentially lying fallow," siloed behind significant security blockades in the interest of preserving their anonymity. So how can both researchers and consumers come out ahead?
Putting Consumers Back in Control
For people who want more control over their genetic data -- plus a share of the proceeds when and if that data is used -- a few companies have paired consumer genomics with blockchain technology to form a new field called "blockchain genomics." Blockchain is a data storage technology that relies on a network of computers, or peer-to-peer setup, making it incredibly difficult to hack. "It's a closed loop of transactions that gets protected and encrypted, and it cannot be changed," says Tanya Woods, a blockchain thought leader and founder of Kind Village, a social impact technology platform.
The vision is to incentivize consumers to share their genomic data and empower researchers to make new breakthroughs.
"So if I agree to give you something and you agree to accept it, we make that exchange, and then that basic framework is captured in a block. … Anything that can be exchanged can be ledgered on blockchain. Anything. It could be real estate, it could be the transfer of artwork, it could be the purchase of a song or any digital content, it could be recognition of a certification," and so on.
The blockchain genomics companies' vision is to incentivize consumers to share their genomic data and empower researchers to make new breakthroughs, all while keeping the data secure and the identities of consumers anonymous.
Consumers, or "partners" as these companies call them, will have a direct say regarding which individuals or organizations can "rent" their data, and will be able to negotiate the amount they receive in exchange. But instead of fiat currency (aka "regular money") as payment, partners will either be remunerated in cryptocurrency unique to the specific company or they will be provided with individual shares of ownership in the database for contributing DNA data and other medical information.
Luna DNA, one of the blockchain genomics companies, "will allow any credible researcher or non-profit to access the databases for a nominal fee," says its president and co-founder, Dawn Barry. Luna DNA's infrastructure was designed to embrace certain conceptions of privacy and privacy law "in which individuals are in total control of their data, including the ability to have their data be 'forgotten' at any time," she said. This is nearly impossible to implement in pre-existing systems that were not designed with full control by the individual in mind.
One of the legal instruments to which Barry referred was the European Union's General Data Protection Regulation, which "states that the data collected on an individual is owned and should be controlled by that individual," she explained. Another is the California Privacy Act that echoes similar principles. "There is a global trend towards more control by the individual that has very deep implications to companies and sites that collect and aggregate data."
David Koepsell, CEO and co-founder of EncrypGen, told Forbes that "Most people are not aware that your DNA contains information about your life expectancy, your proclivity to depression or schizophrenia, your complete ethnic ancestry, your expected intelligence, maybe even your political inclinations" — information that could be misused by insurance companies and employers. And though DTC customers have been assured that their data will stay anonymous, some data can be linked back to consumers' identities. Blockchain may be the answer to these concerns.
Both blockchain technology and the DTC genetic testing arena have a glaring diversity problem.
"The security that's provided by blockchain is tremendous," Woods says. "It's a significant improvement … and as we move toward more digitized economies around the world, these kinds of solutions that are providing security, validity, trust — they're very important."
In the case of blockchain genomics companies like EncrypGen, Luna DNA, Longenesis, and Zenome, each partner who joins would bring a digital copy of their genetic readout from DTC testing companies (like 23andMe or AncestryDNA). The blockchain technology would then be used to record how and for what purposes researchers interact with it. (To learn more about blockchain, check out this helpful visual guide by Reuters.)
Obstacles in the Path to Success
The cryptocurrency approach as a method of payment could be an unattractive lure to consumers if only a limited number of people make transactions in a given currency's network. And the decade-old technology underlying it -- blockchain -- is not yet widely supported, or even well-understood, by the public at large.
"People conflate blockchain with cryptocurrency and bitcoin and all of the concerns and uncertainty thereof," Barry told us. "One can think of cryptocurrency as a single expression of the vast possibilities of the blockchain technology. Blockchain is straightforward in concept and arcane in its implementation."
But blockchain, with its Gini coefficient of 0.98, is one of the most unequal "playing fields" around. The Gini coefficient is a measure of economic inequality, where 0 represents perfect equality and 1 represents perfect inequality. Around 90 percent of bitcoin users, for example, are male, white or Asian, between the ages of 18 and 34, straight, and from middle and upper class families.
The DTC genetic testing arena, too, has a glaring diversity problem. Most DTC genetic test consumers, just like most genetic study participants, are of European descent. In the case of genetic studies, this disparity is largely explained by the fact that most research is done in Europe and North America. In addition to being over 85 percent white, individuals who purchase DTC genetic testing kits are highly educated (about half have more than a college degree), well off (43 percent have a household income of $100,000 or more per year), and are politically liberal (almost 65 percent). Only 14.5 percent of DTC genetic test consumers are non-white, and a mere 5 percent are Hispanic.
Since risk of genetic diseases often varies greatly between ethnic groups, results from DTC tests can be less accurate and less specific for those of non-European ancestry — simply due to a lack of diverse data. The bigger the genetic database, wrote Sarah Zhang for The Atlantic, the more insights 23andMe and other DTC companies "can glean from DNA. That, in turn, means the more [they] can tell customers about their ancestry and health…" Though efforts at recruiting non-white participants have been ongoing, and some successes have been made at improving ancestry tools for people of color, the benefits of genomic gathering in North America are still largely reaped by Caucasians.
So far, it's not yet clear who or how many people will choose to partake in the offerings of blockchain genomics companies.
So one chief hurdle for the blockchain genomics companies is getting the technology into the hands of those who are under-represented in both blockchain and genetic testing research. Women, in particular, may be difficult to bring on board the blockchain genomics bandwagon — though not from lack of interest. Although women make up a significant portion of DTC genetic testing customers (between 50 and 60 percent), their presence is lacking in blockchain and the biotech industry in general.
At the North American Bitcoin Conference in Miami earlier this year, only three women were on stage, compared to 84 men. And the after-party was held in a strip club.
"I was at that conference," Woods told us. "I don't know what happened at the strip club, I didn't observe it. That's not to say it didn't happen … but I enjoyed being at the conference and I enjoyed learning from people who are experimenting in the space and developing in it. Generally, would I have loved to see more women visible? Of course. In tech generally I want to see more women visible, but there's a whole ecosystem shifting that has to happen to make that possible."
Luna's goal is to achieve equal access to a technology (blockchain genomics) that could potentially improve health and quality of life for all involved. But in the merging of two fields that have been unequal since their inception, achieving equal access is one tall order indeed. So far, it's not yet clear who or how many people will choose to participate. LunaDNA's platform has not yet launched; EncrypGen released their beta version just last month.
Sharon Terry, president and CEO of Genetic Alliance — a nonprofit organization that advocates for access to quality genetic services — recently shared a message that reflects the zeitgeist for all those entering the blockchain genomics space: "Be authentic. Tell the truth, even about motives and profits. Be transparent. Engage us. Don't leave us out. Make this real collaboration. Be bold. Take risks. People are dying. It's time to march forward and make a difference."
Doctors worry that fungal pathogens may cause the next pandemic.
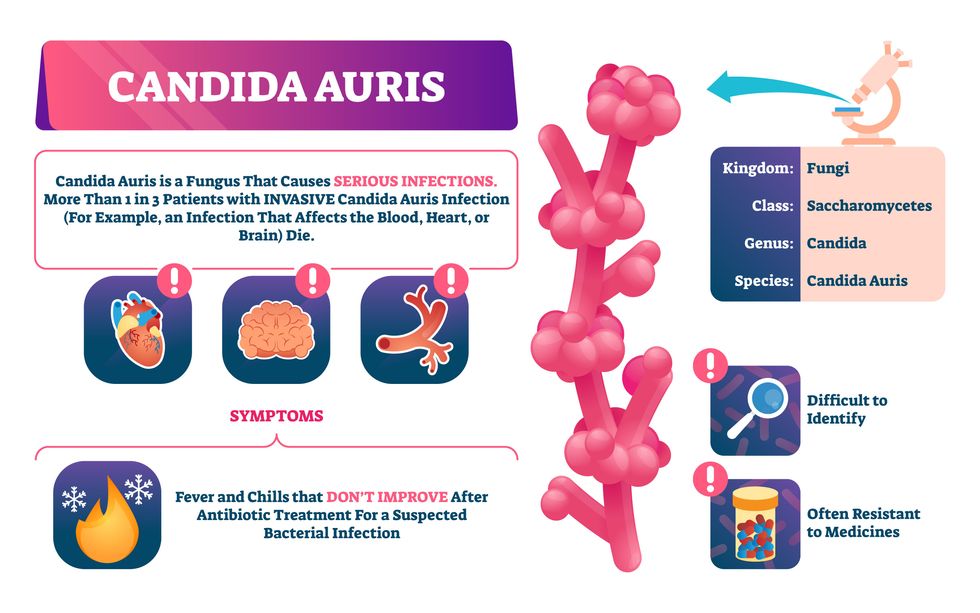
Bacterial antibiotic resistance has been a concern in the medical field for several years. Now a new, similar threat is arising: drug-resistant fungal infections. The Centers for Disease Control and Prevention considers antifungal and antimicrobial resistance to be among the world’s greatest public health challenges.
One particular type of fungal infection caused by Candida auris is escalating rapidly throughout the world. And to make matters worse, C. auris is becoming increasingly resistant to current antifungal medications, which means that if you develop a C. auris infection, the drugs your doctor prescribes may not work. “We’re effectively out of medicines,” says Thomas Walsh, founding director of the Center for Innovative Therapeutics and Diagnostics, a translational research center dedicated to solving the antimicrobial resistance problem. Walsh spoke about the challenges at a Demy-Colton Virtual Salon, one in a series of interactive discussions among life science thought leaders.
Although C. auris typically doesn’t sicken healthy people, it afflicts immunocompromised hospital patients and may cause severe infections that can lead to sepsis, a life-threatening condition in which the overwhelmed immune system begins to attack the body’s own organs. Between 30 and 60 percent of patients who contract a C. auris infection die from it, according to the CDC. People who are undergoing stem cell transplants, have catheters or have taken antifungal or antibiotic medicines are at highest risk. “We’re coming to a perfect storm of increasing resistance rates, increasing numbers of immunosuppressed patients worldwide and a bug that is adapting to higher temperatures as the climate changes,” says Prabhavathi Fernandes, chair of the National BioDefense Science Board.
Most Candida species aren’t well-adapted to our body temperatures so they aren’t a threat. C. auris, however, thrives at human body temperatures.
Although medical professionals aren’t concerned at this point about C. auris evolving to affect healthy people, they worry that its presence in hospitals can turn routine surgeries into life-threatening calamities. “It’s coming,” says Fernandes. “It’s just a matter of time.”
An emerging global threat
“Fungi are found in the environment,” explains Fernandes, so Candida spores can easily wind up on people’s skin. In hospitals, they can be transferred from contact with healthcare workers or contaminated surfaces. Most Candida species aren’t well-adapted to our body temperatures so they aren’t a threat. C. auris, however, thrives at human body temperatures. It can enter the body during medical treatments that break the skin—and cause an infection. Overall, fungal infections cost some $48 billion in the U.S. each year. And infection rates are increasing because, in an ironic twist, advanced medical therapies are enabling severely ill patients to live longer and, therefore, be exposed to this pathogen.
The first-ever case of a C. auris infection was reported in Japan in 2009, although an analysis of Candida samples dated the earliest strain to a 1996 sample from South Korea. Since then, five separate varieties – called clades, which are similar to strains among bacteria – developed independently in different geographies: South Asia, East Asia, South Africa, South America and, recently, Iran. So far, C. auris infections have been reported in 35 countries.
In the U.S., the first infection was reported in 2016, and the CDC started tracking it nationally two years later. During that time, 5,654 cases have been reported to the CDC, which only tracks U.S. data.
What’s more notable than the number of cases is their rate of increase. In 2016, new cases increased by 175 percent and, on average, they have approximately doubled every year. From 2016 through 2022, the number of infections jumped from 63 to 2,377, a roughly 37-fold increase.
“This reminds me of what we saw with epidemics from 2013 through 2020… with Ebola, Zika and the COVID-19 pandemic,” says Robin Robinson, CEO of Spriovas and founding director of the Biomedical Advanced Research and Development Authority (BARDA), which is part of the U.S. Department of Health and Human Services. These epidemics started with a hockey stick trajectory, Robinson says—a gradual growth leading to a sharp spike, just like the shape of a hockey stick.
Another challenge is that right now medics don’t have rapid diagnostic tests for fungal infections. Currently, patients are often misdiagnosed because C. auris resembles several other easily treated fungi. Or they are diagnosed long after the infection begins and is harder to treat.
The problem is that existing diagnostics tests can only identify C. auris once it reaches the bloodstream. Yet, because this pathogen infects bodily tissues first, it should be possible to catch it much earlier before it becomes life-threatening. “We have to diagnose it before it reaches the bloodstream,” Walsh says.
The most alarming fact is that some Candida infections no longer respond to standard therapeutics.
“We need to focus on rapid diagnostic tests that do not rely on a positive blood culture,” says John Sperzel, president and CEO of T2 Biosystems, a company specializing in diagnostics solutions. Blood cultures typically take two to three days for the concentration of Candida to become large enough to detect. The company’s novel test detects about 90 percent of Candida species within three to five hours—thanks to its ability to spot minute quantities of the pathogen in blood samples instead of waiting for them to incubate and proliferate.
Unlike other Candida species C. auris thrives at human body temperatures
Adobe Stock
Tackling the resistance challenge
The most alarming fact is that some Candida infections no longer respond to standard therapeutics. The number of cases that stopped responding to echinocandin, the first-line therapy for most Candida infections, tripled in 2020, according to a study by the CDC.
Now, each of the first four clades shows varying levels of resistance to all three commonly prescribed classes of antifungal medications, such as azoles, echinocandins, and polyenes. For example, 97 percent of infections from C. auris Clade I are resistant to fluconazole, 54 percent to voriconazole and 30 percent of amphotericin. Nearly half are resistant to multiple antifungal drugs. Even with Clade II fungi, which has the least resistance of all the clades, 11 to 14 percent have become resistant to fluconazole.
Anti-fungal therapies typically target specific chemical compounds present on fungi’s cell membranes, but not on human cells—otherwise the medicine would cause damage to our own tissues. Fluconazole and other azole antifungals target a compound called ergosterol, preventing the fungal cells from replicating. Over the years, however, C. auris evolved to resist it, so existing fungal medications don’t work as well anymore.
A newer class of drugs called echinocandins targets a different part of the fungal cell. “The echinocandins – like caspofungin – inhibit (a part of the fungi) involved in making glucan, which is an essential component of the fungal cell wall and is not found in human cells,” Fernandes says. New antifungal treatments are needed, she adds, but there are only a few magic bullets that will hit just the fungus and not the human cells.
Research to fight infections also has been challenged by a lack of government support. That is changing now that BARDA is requesting proposals to develop novel antifungals. “The scope includes C. auris, as well as antifungals following a radiological/nuclear emergency, says BARDA spokesperson Elleen Kane.
The remaining challenge is the number of patients available to participate in clinical trials. Large numbers are needed, but the available patients are quite sick and often die before trials can be completed. Consequently, few biopharmaceutical companies are developing new treatments for C. auris.
ClinicalTrials.gov reports only two drugs in development for invasive C. auris infections—those than can spread throughout the body rather than localize in one particular area, like throat or vaginal infections: ibrexafungerp by Scynexis, Inc., fosmanogepix, by Pfizer.
Scynexis’ ibrexafungerp appears active against C. auris and other emerging, drug-resistant pathogens. The FDA recently approved it as a therapy for vaginal yeast infections and it is undergoing Phase III clinical trials against invasive candidiasis in an attempt to keep the infection from spreading.
“Ibreafungerp is structurally different from other echinocandins,” Fernandes says, because it targets a different part of the fungus. “We’re lucky it has activity against C. auris.”
Pfizer’s fosmanogepix is in Phase II clinical trials for patients with invasive fungal infections caused by multiple Candida species. Results are showing significantly better survival rates for people taking fosmanogepix.
Although C. auris does pose a serious threat to healthcare worldwide, scientists try to stay optimistic—because they recognized the problem early enough, they might have solutions in place before the perfect storm hits. “There is a bit of hope,” says Robinson. “BARDA has finally been able to fund the development of new antifungal agents and, hopefully, this year we can get several new classes of antifungals into development.”
New elevators could lift up our access to space
A space elevator would be cheaper and cleaner than using rockets
Story by Big Think
When people first started exploring space in the 1960s, it cost upwards of $80,000 (adjusted for inflation) to put a single pound of payload into low-Earth orbit.
A major reason for this high cost was the need to build a new, expensive rocket for every launch. That really started to change when SpaceX began making cheap, reusable rockets, and today, the company is ferrying customer payloads to LEO at a price of just $1,300 per pound.
This is making space accessible to scientists, startups, and tourists who never could have afforded it previously, but the cheapest way to reach orbit might not be a rocket at all — it could be an elevator.
The space elevator
The seeds for a space elevator were first planted by Russian scientist Konstantin Tsiolkovsky in 1895, who, after visiting the 1,000-foot (305 m) Eiffel Tower, published a paper theorizing about the construction of a structure 22,000 miles (35,400 km) high.
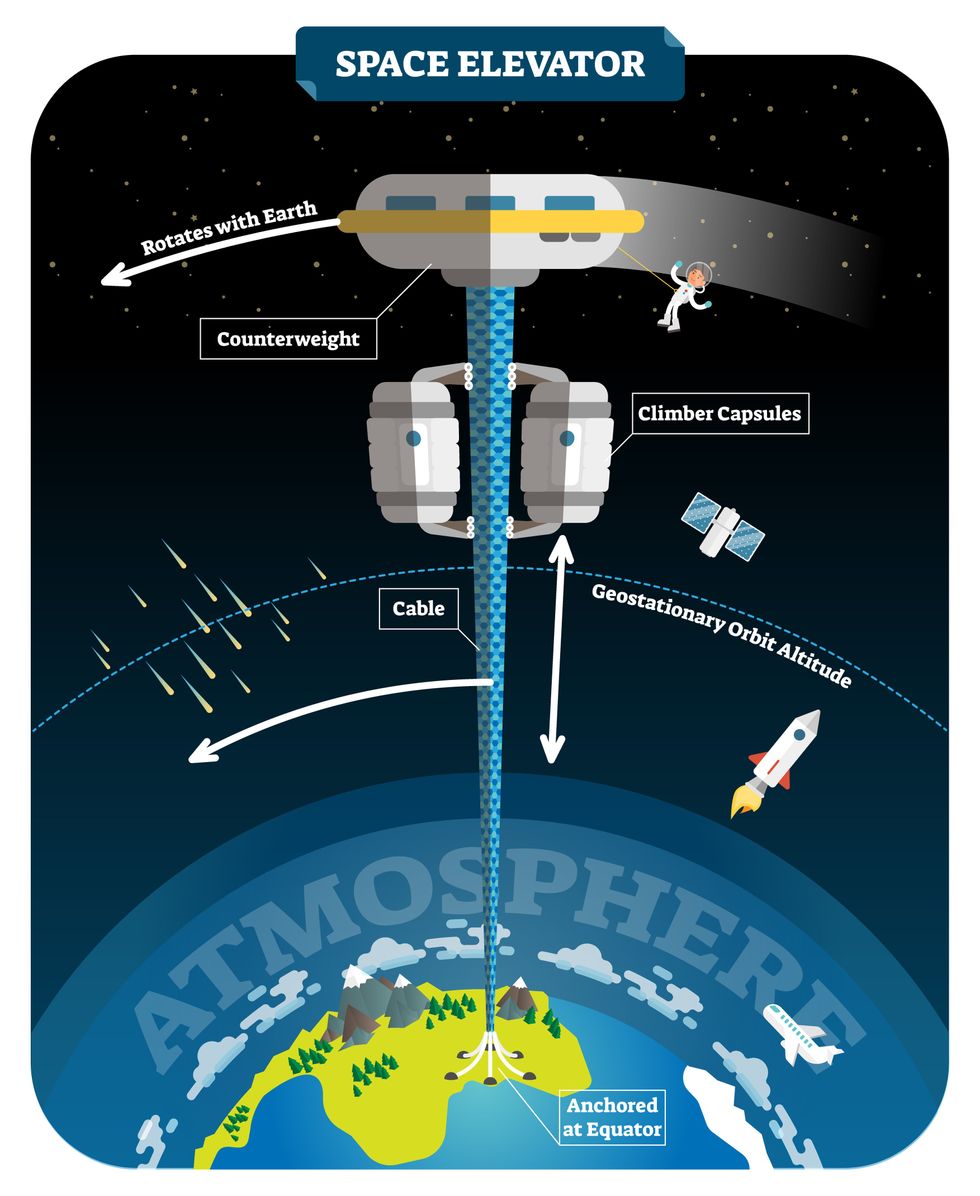
This would provide access to geostationary orbit, an altitude where objects appear to remain fixed above Earth’s surface, but Tsiolkovsky conceded that no material could support the weight of such a tower.
We could then send electrically powered “climber” vehicles up and down the tether to deliver payloads to any Earth orbit.
In 1959, soon after Sputnik, Russian engineer Yuri N. Artsutanov proposed a way around this issue: instead of building a space elevator from the ground up, start at the top. More specifically, he suggested placing a satellite in geostationary orbit and dropping a tether from it down to Earth’s equator. As the tether descended, the satellite would ascend. Once attached to Earth’s surface, the tether would be kept taut, thanks to a combination of gravitational and centrifugal forces.
We could then send electrically powered “climber” vehicles up and down the tether to deliver payloads to any Earth orbit. According to physicist Bradley Edwards, who researched the concept for NASA about 20 years ago, it’d cost $10 billion and take 15 years to build a space elevator, but once operational, the cost of sending a payload to any Earth orbit could be as low as $100 per pound.
“Once you reduce the cost to almost a Fed-Ex kind of level, it opens the doors to lots of people, lots of countries, and lots of companies to get involved in space,” Edwards told Space.com in 2005.
In addition to the economic advantages, a space elevator would also be cleaner than using rockets — there’d be no burning of fuel, no harmful greenhouse emissions — and the new transport system wouldn’t contribute to the problem of space junk to the same degree that expendable rockets do.
So, why don’t we have one yet?
Tether troubles
Edwards wrote in his report for NASA that all of the technology needed to build a space elevator already existed except the material needed to build the tether, which needs to be light but also strong enough to withstand all the huge forces acting upon it.
The good news, according to the report, was that the perfect material — ultra-strong, ultra-tiny “nanotubes” of carbon — would be available in just two years.
“[S]teel is not strong enough, neither is Kevlar, carbon fiber, spider silk, or any other material other than carbon nanotubes,” wrote Edwards. “Fortunately for us, carbon nanotube research is extremely hot right now, and it is progressing quickly to commercial production.”Unfortunately, he misjudged how hard it would be to synthesize carbon nanotubes — to date, no one has been able to grow one longer than 21 inches (53 cm).
Further research into the material revealed that it tends to fray under extreme stress, too, meaning even if we could manufacture carbon nanotubes at the lengths needed, they’d be at risk of snapping, not only destroying the space elevator, but threatening lives on Earth.
Looking ahead
Carbon nanotubes might have been the early frontrunner as the tether material for space elevators, but there are other options, including graphene, an essentially two-dimensional form of carbon that is already easier to scale up than nanotubes (though still not easy).
Contrary to Edwards’ report, Johns Hopkins University researchers Sean Sun and Dan Popescu say Kevlar fibers could work — we would just need to constantly repair the tether, the same way the human body constantly repairs its tendons.
“Using sensors and artificially intelligent software, it would be possible to model the whole tether mathematically so as to predict when, where, and how the fibers would break,” the researchers wrote in Aeon in 2018.
“When they did, speedy robotic climbers patrolling up and down the tether would replace them, adjusting the rate of maintenance and repair as needed — mimicking the sensitivity of biological processes,” they continued.Astronomers from the University of Cambridge and Columbia University also think Kevlar could work for a space elevator — if we built it from the moon, rather than Earth.
They call their concept the Spaceline, and the idea is that a tether attached to the moon’s surface could extend toward Earth’s geostationary orbit, held taut by the pull of our planet’s gravity. We could then use rockets to deliver payloads — and potentially people — to solar-powered climber robots positioned at the end of this 200,000+ mile long tether. The bots could then travel up the line to the moon’s surface.
This wouldn’t eliminate the need for rockets to get into Earth’s orbit, but it would be a cheaper way to get to the moon. The forces acting on a lunar space elevator wouldn’t be as strong as one extending from Earth’s surface, either, according to the researchers, opening up more options for tether materials.
“[T]he necessary strength of the material is much lower than an Earth-based elevator — and thus it could be built from fibers that are already mass-produced … and relatively affordable,” they wrote in a paper shared on the preprint server arXiv.
After riding up the Earth-based space elevator, a capsule would fly to a space station attached to the tether of the moon-based one.
Electrically powered climber capsules could go up down the tether to deliver payloads to any Earth orbit.
Adobe Stock
Some Chinese researchers, meanwhile, aren’t giving up on the idea of using carbon nanotubes for a space elevator — in 2018, a team from Tsinghua University revealed that they’d developed nanotubes that they say are strong enough for a tether.
The researchers are still working on the issue of scaling up production, but in 2021, state-owned news outlet Xinhua released a video depicting an in-development concept, called “Sky Ladder,” that would consist of space elevators above Earth and the moon.
After riding up the Earth-based space elevator, a capsule would fly to a space station attached to the tether of the moon-based one. If the project could be pulled off — a huge if — China predicts Sky Ladder could cut the cost of sending people and goods to the moon by 96 percent.
The bottom line
In the 120 years since Tsiolkovsky looked at the Eiffel Tower and thought way bigger, tremendous progress has been made developing materials with the properties needed for a space elevator. At this point, it seems likely we could one day have a material that can be manufactured at the scale needed for a tether — but by the time that happens, the need for a space elevator may have evaporated.
Several aerospace companies are making progress with their own reusable rockets, and as those join the market with SpaceX, competition could cause launch prices to fall further.
California startup SpinLaunch, meanwhile, is developing a massive centrifuge to fling payloads into space, where much smaller rockets can propel them into orbit. If the company succeeds (another one of those big ifs), it says the system would slash the amount of fuel needed to reach orbit by 70 percent.
Even if SpinLaunch doesn’t get off the ground, several groups are developing environmentally friendly rocket fuels that produce far fewer (or no) harmful emissions. More work is needed to efficiently scale up their production, but overcoming that hurdle will likely be far easier than building a 22,000-mile (35,400-km) elevator to space.
This article originally appeared on Big Think, home of the brightest minds and biggest ideas of all time.


