Your Prescription Is Ready for Download

A close up of a doctor pointing at a smart phone, heralding the new era of prescription digital therapeutics.
You may be familiar with Moore's Law, the prediction made by Intel co-founder Gordon Moore that computer chips would get faster and cheaper with each passing year. That's been borne out by the explosive growth of the tech industry, but you may not know that there is an inverse Moore's Law for drug development.
What if there were a way to apply the fast-moving, low-cost techniques of software development to drug discovery?
Eroom's Law—yes that's "Moore" spelled backward—is the observation that drug discovery has become slower and more expensive over time, despite technological improvements. And just like Moore's Law, it's been borne out by experience—from the 1950s to today, the number of drugs that can be developed per billion dollars in spending has steadily decreased, contributing to the continued growth of health care costs.
But what if there were a way to apply the fast-moving, low-cost techniques of software development to drug discovery? That's what a group of startups in the new field of digital therapeutics are promising. They develop apps that are used—either on their own or in conjunction with conventional drugs—to treat chronic disorders like addiction, diabetes and mental health that have so far resisted a pharmaceutical approach. Unlike the thousands of wellness and health apps that can be downloaded to your phone, digital therapeutics are developed and are meant to be used like drugs, complete with clinical trials, FDA approval and doctor prescriptions.
The field is hot—in 2017 global investment in digital therapeutics jumped to $11.5 billion, a fivefold increase from 2012, and major pharma companies like Novartis are developing their own digital products or partnering with startups. One such startup is the bicoastal Pear Therapeutics. Last month, Pear's reSET-O product became the first digital therapeutic to be approved for use by the millions of Americans who struggle with opioid use disorder, and the company has other products addressing addiction and mental illness in the pipeline.
I spoke with Dr. Corey McCann, Pear's CEO, about the company's efforts to meld software and medicine, designing clinical trials for an entirely new kind of treatment, and the future of digital therapeutics.
The interview has been edited and condensed for clarity and length.
"We're looking at conditions that currently can't be cured with drugs."
BRYAN WALSH: What makes a digital therapeutic different than a wellness app?
COREY MCCANN: What we do is develop therapeutics that are designed to be used under the auspices of a physician, just as a drug developed under good manufacturing would be. We do clinical studies for both safety and efficacy, and then they go through the development process you'd expect for a drug. We look at the commercial side, at the role of doctors. Everything we do is what would be done with a traditional medical product. It's a piece of software developed like a drug.
WALSH: What kind of conditions are you first aiming to treat with digital therapeutics?
MCCANN: We're looking at conditions that currently can't be cured with drugs. A good example is our reSET product, which is designed to treat addiction to alcohol, cannabis, stimulants, cocaine. There really aren't pharmaceutical products that are approved to treat people addicted to these substances. What we're doing is functional therapy, the standard of care for addiction treatment, but delivered via software. But we can also work with medication—our reSET-O product is a great example. It's for patients struggling with opioid addiction, and it's delivered in concert with the drug buprenorphine.
WALSH: Walk me through what the patient experience would be like for someone on a digital therapeutic like reSET.
MCCANN: Imagine you're a patient who has been diagnosed with cocaine addiction by a doctor. You would then receive a prescription for reSET during the same office visit. Instead of a pharmacy, the script is sent to the reSET Connect Patient Service Center, where you are onboarded and given an access code that is used to unlock the product after downloading it onto your device. The product has 60 different modules—each one requiring about a 10 to 15-minute interaction—all derived from a form of cognitive behavioral therapy called community reinforcement approach. The treatment takes place over 90 days.
"The patients receiving the digital therapeutic were more than twice as likely to remain abstinent as those receiving standard care."
Patients report their substance abuse, cravings and triggers, and they are also tested on core proficiencies through the therapy. Physicians have access to all of their data, which helps facilitate their one-on-one meetings. We know from regular urine tests how effective the treatment is.
WALSH: What kind of data did you find when you did clinical studies on reSET?
MCCANN: We had 399 patients in 10 centers taking part in a randomized clinical trial run by the National Institute on Drug Abuse. Every patient enrolled in the study had an active substance abuse disorder. The study was randomized so that patients either received the best current standard of care, which is three hours a week of face-to-face therapy, or they received the digital therapeutic. The primary endpoint was abstinence in weeks 9 to 12—if the patient had a single dirty urine screen in the last month, they counted as a failure.
In the end, the patients receiving the digital therapeutic were more than twice as likely to remain abstinent as those receiving standard care—40 percent versus 17 percent. Those receiving reSET were also much more likely to remain in treatment through the entire trial.
WALSH: Why start by focusing your first digital therapeutics on addiction?
MCCANN: We have tried to build a company that is poised to make a difference in medicine. If you look at addiction, there is little to nothing in the drug pipeline to address this. More than 30 million people in the U.S. suffer from addiction disorders, and not only is efficacy a concern, but so is access. Many patients aren't able to receive anything like the kind of face-to-face therapy our control group received. So we think digital therapeutics can make a difference there as well.
WALSH: reSET was the first digital therapeutic approved by the FDA to treat a specific disorder. What has the approval process been like?
MCCANN: It's been a learning process for all involved, including the FDA. Our philosophy is to work within the clinical trials structure, which has specific disease targets and endpoints, and develop quality software, and bring those two strands together to generate digital therapeutics. We now have two products that have been FDA-approved, and four more in development. The FDA is appropriately cautious about all of this, balancing the tradeoff between patient risk and medical value. As we see it, our company is half tech and half biotech, and we follow regulatory trials that are as rigorous as they would be with any drug company.
"This is a new space, but when you look back in 10 years there will be an entire industry of prescription digital therapeutics."
WALSH: How do you balance those two halves, the tech side and the biology side? Tech companies are known for iterating rapidly and cheaply, while pharma companies develop drugs slowly and expensively.
MCCANN: This is a new space, but when you look back in 10 years there will be an entire industry of prescription digital therapeutics. Right now for us we're combining the rigor of the pharmaceutical model with the speed and agility of a tech company. Our product takes longer to develop than an unverified health app, but less time and with less clinical risk than a new molecular entity. This is still a work in progress and not a day goes by where we don't notice the difference between those disciplines.
WALSH: Who's going to pay for these treatments? Insurers are traditionally slow to accept new innovations in the therapeutic space.
MCCANN: This is just like any drug launch. We need to show medical quality and value, and we need to get clinician demand. We want to focus on demonstrating as many scripts as we can in 2019. And we know we'll need to be persistent—we live in a world where payers will say no to anything three times before they say yes. Demonstrating value is how you get there.
WALSH: Is part of that value the possibility that digital therapeutics could be much cheaper than paying someone for multiple face-to-face therapy sessions?
MCCANN: I believe the cost model is very compelling here, especially when you can treat diseases that were not treatable before. That is something that creates medical value. Then you have the data aspect, which makes our product fundamentally different from a drug. We know everything about every patient that uses our product. We know engagement, we can push patient self-reports to clinicians. We can measure efficiency out in the real world, not just in a measured clinical trial. That is the holy grail in the pharma world—to understand compliance in practice.
WALSH: What's the future of digital therapeutics?
MCCANN: In 10 years, what we think of as digital medicine will just be medicine. This is something that will absolutely become standard of care. We are working on education to help partners and payers figure out where go from here, and to incorporate digital therapeutics into standard care. It will start in 2019 and 2020 with addiction medicine, and then in three to five years you'll see treatments designed to address disorders of the brain. And then past the decade horizon you'll see plenty of products that aim at every facet of medicine.
Tech-related injuries are becoming more common as many people depend on - and often develop addictions for - smart phones and computers.
In the 1990s, a mysterious virus spread throughout the Massachusetts Institute of Technology Artificial Intelligence Lab—or that’s what the scientists who worked there thought. More of them rubbed their aching forearms and massaged their cricked necks as new computers were introduced to the AI Lab on a floor-by-floor basis. They realized their musculoskeletal issues coincided with the arrival of these new computers—some of which were mounted high up on lab benches in awkward positions—and the hours spent typing on them.
Today, these injuries have become more common in a society awash with smart devices, sleek computers, and other gadgets. And we don’t just get hurt from typing on desktop computers; we’re massaging our sore wrists from hours of texting and Facetiming on phones, especially as they get bigger in size.
In 2007, the first iPhone measured 3.5-inches diagonally, a measurement known as the display size. That’s been nearly doubled by the newest iPhone 13 Pro, which has a 6.7-inch display. Other phones, too, like the Google Pixel 6 and the Samsung Galaxy S22, have bigger screens than their predecessors. Physical therapists and orthopedic surgeons have had to come up with names for a variety of new conditions: selfie elbow, tech neck, texting thumb. Orthopedic surgeon Sonya Sloan says she sees selfie elbow in younger kids and in women more often than men. She hears complaints related to technology once or twice a day.
The addictive quality of smartphones and social media means that people spend more time on their devices, which exacerbates injuries. According to Statista, 68 percent of those surveyed spent over three hours a day on their phone, and almost half spent five to six hours a day. Another report showed that people dedicate a third of their day to checking their phones, while the Media Effects Research Laboratory at Pennsylvania State University has found that bigger screens, ideal for entertainment purposes, immerse their users more than smaller screens. Oversized screens also provide easier navigation and more space for those with bigger hands or trouble seeing.
But others with conditions like arthritis can benefit from smaller phones. In March of 2016, Apple released the iPhone SE with a display size of 4.7 inches—an inch smaller than the iPhone 7, released that September. Apple has since come out with two more versions of the diminutive iPhone SE, one in 2020 and another in 2022.
These devices are now an inextricable part of our lives. So where does the burden of responsibility lie? Is it with consumers to adjust body positioning, get ergonomic workstations, and change habits to abate tech-related pain? Or should tech companies be held accountable?
Kavin Senapathy, a freelance science journalist, has the Google Pixel 6. She was drawn to the phone because Google marketed the Pixel 6’s camera as better at capturing different skin tones. But this phone boasts one of the largest display sizes on the market: 6.4 inches.
Senapathy was diagnosed with carpal and cubital tunnel syndromes in 2017 and fibromyalgia in 2019. She has had to create a curated ergonomic workplace setup, otherwise her wrists and hands get weak and tingly, and she’s had to adjust how she holds her phone to prevent pain flares.
Recently, Senapathy underwent an electromyography, or an EMG, in which doctors insert electrodes into muscles to measure their electrical activity. The electrical response of the muscles tells doctors whether the nerve cells and muscles are successfully communicating. Depending on her results, steroid shots and even surgery might be required. Senapathy wants to stick with her Pixel 6, but the pain she’s experiencing may push her to buy a smaller phone. Unfortunately, options for these modestly sized phones are more limited.
These devices are now an inextricable part of our lives. So where does the burden of responsibility lie? Is it with consumers like Senapathy to adjust body positioning, get ergonomic workstations, and change habits to abate tech-related pain? Or should tech companies be held accountable for creating addictive devices that lead to musculoskeletal injury?

Kavin Senapathy, a freelance journalist, bought the Google Pixel 6 because of its high-quality camera, but she’s had to adjust how she holds the oversized phone to prevent pain flares.
Kavin Senapathy
A one-size-fits-all mentality for smartphones will continue to lead to injuries because every user has different wants and needs. S. Shyam Sundar, the founder of Penn State’s lab on media effects and a communications professor, says the needs for mobility and portability conflict with the desire for greater visibility. “The best thing a company can do is offer different sizes,” he says.
Joanna Bryson, an AI ethics expert and professor at The Hertie School of Governance in Berlin, Germany, echoed these sentiments. “A lot of the lack of choice we see comes from the fact that the markets have consolidated so much,” she says. “We want to make sure there’s sufficient diversity [of products].”
Consumers can still maintain some control despite the ubiquity of tech. Sloan, the orthopedic surgeon, has to pester her son to change his body positioning when using his tablet. Our heads get heavier as they bend forward: at rest, they weigh 12 pounds, but bent 60 degrees, they weigh 60. “I have to tell him, ‘Raise your head, son!’” she says. It’s important, Sloan explains, to consider that growth and development will affect ligaments and bones in the neck, potentially making kids even more vulnerable to injuries from misusing gadgets. She recommends that parents limit their kids’ tech time to alleviate strain. She also suggested that tech companies implement a timer to remind us to change our body positioning.
In 2017, Nan-Wei Gong, a former contractor for Google, founded Figur8, which uses wearable trackers to measure muscle function and joint movement. It’s like physical therapy with biofeedback. “Each unique injury has a different biomarker,” says Gong. “With Figur8, you are comparing yourself to yourself.” This allows an individual to self-monitor for wear and tear and strengthen an injury in a way that’s efficient and designed for their body. Gong noticed that the work-from-home model during the COVID-19 pandemic created a new set of ergonomic problems that resulted in injuries. Figur8 provides real-time data for these injuries because “behavioral change requires feedback.”
Gong worked on a project called Jacquard while at Google. Textile experts weave conductive thread into their fabric, and the result is a patch of the fabric—like the cuff of a Levi’s jacket—that responds to commands on your smartphone. One swipe can call your partner or check the weather. It was designed with cyclists in mind who can’t easily check their phones, and it’s part of a growing movement in the tech industry to deliver creative, hands-free design. Gong thinks that engineers at large corporations like Google have accessibility in mind; it’s part of what drives their decisions for new products.
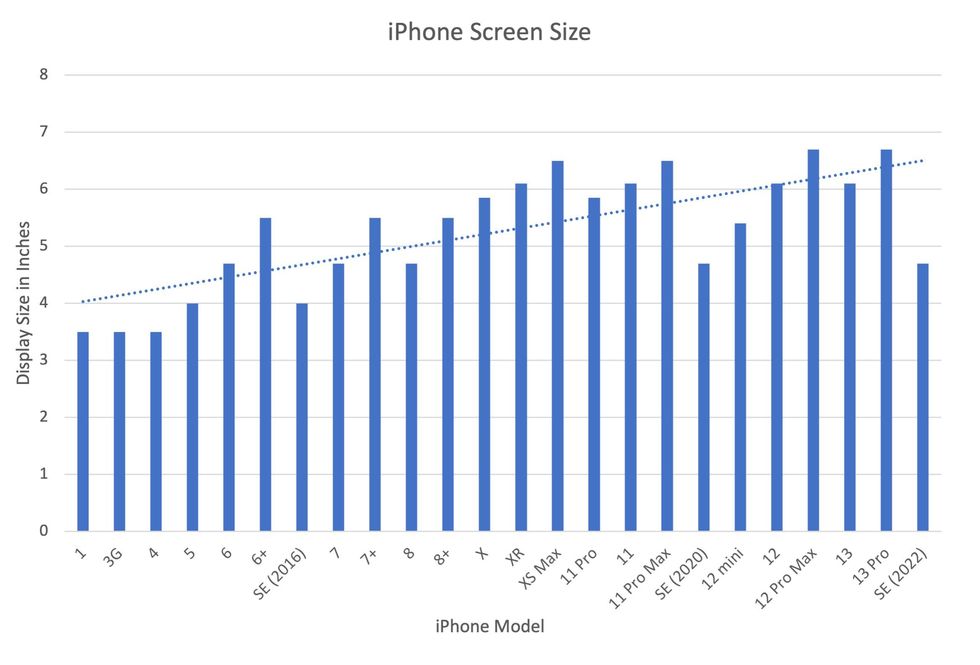
Display sizes of iPhones have become larger over time.
Sourced from Screenrant https://screenrant.com/iphone-apple-release-chronological-order-smartphone/ and Apple Tech Specs: https://www.apple.com/iphone-se/specs/
Back in Germany, Joanna Bryson reminds us that products like smartphones should adhere to best practices. These rules may be especially important for phones and other products with AI that are addictive. Disclosure, accountability, and regulation are important for AI, she says. “The correct balance will keep changing. But we have responsibilities and obligations to each other.” She was on an AI Ethics Council at Google, but the committee was disbanded after only one week due to issues with one of their members.
Bryson was upset about the Council’s dissolution but has faith that other regulatory bodies will prevail. OECD.AI, and international nonprofit, has drafted policies to regulate AI, which countries can sign and implement. “As of July 2021, 46 governments have adhered to the AI principles,” their website reads.
Sundar, the media effects professor, also directs Penn State’s Center for Socially Responsible AI. He says that inclusivity is a crucial aspect of social responsibility and how devices using AI are designed. “We have to go beyond first designing technologies and then making them accessible,” he says. “Instead, we should be considering the issues potentially faced by all different kinds of users before even designing them.”
Entomologist Jessica Ware is using new technologies to identify insect species in a changing climate. She shares her suggestions for how we can live harmoniously with creeper crawlers everywhere.
Jessica Ware is obsessed with bugs.
My guest today is a leading researcher on insects, the president of the Entomological Society of America and a curator at the American Museum of Natural History. Learn more about her here.
You may not think that insects and human health go hand-in-hand, but as Jessica makes clear, they’re closely related. A lot of people care about their health, and the health of other creatures on the planet, and the health of the planet itself, but researchers like Jessica are studying another thing we should be focusing on even more: how these seemingly separate areas are deeply entwined. (This is the theme of an upcoming event hosted by Leaps.org and the Aspen Institute.)
Listen to the Episode
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google

Entomologist Jessica Ware
D. Finnin / AMNH
Maybe it feels like a core human instinct to demonize bugs as gross. We seem to try to eradicate them in every way possible, whether that’s with poison, or getting out our blood thirst by stomping them whenever they creep and crawl into sight.
But where did our fear of bugs really come from? Jessica makes a compelling case that a lot of it is cultural, rather than in-born, and we should be following the lead of other cultures that have learned to live with and appreciate bugs.
The truth is that a healthy planet depends on insects. You may feel stung by that news if you hate bugs. Reality bites.
Jessica and I talk about whether learning to live with insects should include eating them and gene editing them so they don’t transmit viruses. She also tells me about her important research into using genomic tools to track bugs in the wild to figure out why and how we’ve lost 50 percent of the insect population since 1970 according to some estimates – bad news because the ecosystems that make up the planet heavily depend on insects. Jessica is leading the way to better understand what’s causing these declines in order to start reversing these trends to save the insects and to save ourselves.

