Arguing About Vaccines Doesn’t Usually Work — But This Might
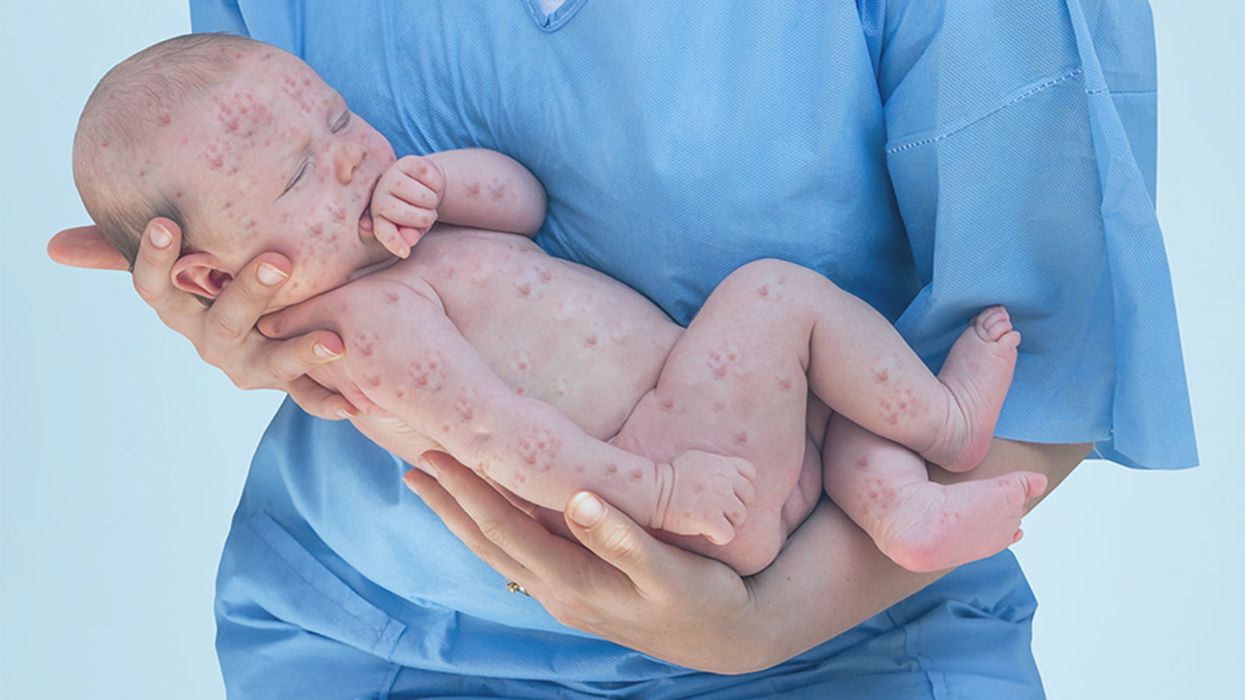
A doctor cradles a newborn who is sick with measles.
Ethan Lindenberger, the Ohio teenager who sought out vaccinations after he was denied them as a child, recently testified before Congress about why his parents became anti-vaxxers. The trouble, he believes, stems from the pervasiveness of misinformation online.
There is evidence that 'educating' people with facts about the benefits of vaccination may not be effective.
"For my mother, her love and affection and care as a parent was used to push an agenda to create a false distress," he told the Senate Committee. His mother read posts on social media saying vaccines are dangerous, and that was enough to persuade her against them.
His story is an example of how widespread and harmful the current discourse on vaccinations is—and more importantly—how traditional strategies to convince people about the merits of vaccination have largely failed.
As responsible members of society, all of us have implicitly signed on to what ethicists call the "Social Contract" -- we agree to abide by certain moral and political rules of behavior. This is what our societal values, norms, and often governments are based upon. However, with the unprecedented rise of social media, alternative facts, and fake news, it is evident that our understanding—and application—of the social contract must also evolve.
Nowhere is this breakdown of societal norms more visible than in the failure to contain the spread of vaccine-preventable diseases like measles. What started off as unexplained episodes in New York City last October, mostly in communities that are under-vaccinated, has exploded into a national epidemic: 880 cases of measles across 24 states in 2019, according to the CDC (as of May 17, 2019). In fact, the Unites States is only eight months away from losing its "measles free" status, joining Venezuela as the second country out of North and South America with that status.
The U.S. is not the only country facing this growing problem. Such constant and perilous reemergence of measles and other vaccine-preventable diseases in various parts of the world raises doubts about the efficacy of current vaccination policies. In addition to the loss of valuable life, these outbreaks lead to loss of millions of dollars in unnecessary expenditure of scarce healthcare resources. While we may be living through an age of information, we are also navigating an era whose hallmark is a massive onslaught on truth.
There is ample evidence on how these outbreaks start: low-vaccination rates. At the same time, there is evidence that 'educating' people with facts about the benefits of vaccination may not be effective. Indeed, human reasoning has a limit, and facts alone rarely change a person's opinion. In a fascinating report by researchers from the University of Pennsylvania, a small experiment revealed how "behavioral nudges" could inform policy decisions around vaccination.
In the reported experiment, the vaccination rate for employees of a company increased by 1.5 percent when they were prompted to name the date when they planned to get their flu shot. In the same experiment, when employees were prompted to name both a date and a time for their planned flu shot, vaccination rate increased by 4 percent.
A randomized trial revealed the subtle power of "announcements" – direct, brief, assertive statements by physicians that assumed parents were ready to vaccinate their children.
This experiment is a part of an emerging field of behavioral economics—a scientific undertaking that uses insights from psychology to understand human decision-making. The field was born from a humbling realization that humans probably do not possess an unlimited capacity for processing information. Work in this field could inform how we can formulate vaccination policy that is effective, conserves healthcare resources, and is applicable to current societal norms.
Take, for instance, the case of Human Papilloma Virus (HPV) that can cause several types of cancers in both men and women. Research into the quality of physician communication has repeatedly revealed how lukewarm recommendations for HPV vaccination by primary care physicians likely contributes to under-immunization of eligible adolescents and can cause confusion for parents.
A randomized trial revealed the subtle power of "announcements" – direct, brief, assertive statements by physicians that assumed parents were ready to vaccinate their children. These announcements increased vaccination rates by 5.4 percent. Lengthy, open-ended dialogues demonstrated no benefit in vaccination rates. It seems that uncertainty from the physician translates to unwillingness from a parent.
Choice architecture is another compelling concept. The premise is simple: We hardly make any of our decisions in vacuum; the environment in which these decisions are made has an influence. If health systems were designed with these insights in mind, people would be more likely to make better choices—without being forced.
This theory, proposed by Richard Thaler, who won the 2017 Nobel Prize in Economics, was put to the test by physicians at the University of Pennsylvania. In their study, flu vaccination rates at primary care practices increased by 9.5 percent all because the staff implemented "active choice intervention" in their electronic health records—a prompt that nudged doctors and nurses to ask patients if they'd gotten the vaccine yet. This study illustrated how an intervention as simple as a reminder can save lives.
To be sure, some bioethicists do worry about implementing these policies. Are behavioral nudges akin to increased scrutiny or a burden for the disadvantaged? For example, would incentives to quit smoking unfairly target the poor, who are more likely to receive criticism for bad choices?
The measles outbreak is a sober reminder of how devastating it can be when the social contract breaks down.
While this is a valid concern, behavioral economics offers one of the only ethical solutions to increasing vaccination rates by addressing the most critical—and often legal—challenge to universal vaccinations: mandates. Choice architecture and other interventions encourage and inform a choice, allowing an individual to retain his or her right to refuse unwanted treatment. This distinction is especially important, as evidence suggests that people who refuse vaccinations often do so as a result of cognitive biases – systematic errors in thinking resulting from emotional attachment or a lack of information.
For instance, people are prone to "confirmation bias," or a tendency to selectively believe in information that confirms their preexisting theories, rather than the available evidence. At the same time, people do not like mandates. In such situations, choice architecture provides a useful option: people are nudged to make the right choice via the design of health delivery systems, without needing policies that rely on force.
The measles outbreak is a sober reminder of how devastating it can be when the social contract breaks down and people fall prey to misinformation. But all is not lost. As we fight a larger societal battle against alternative facts, we now have another option in the trenches to subtly encourage people to make better choices.
Using insights from research in decision-making, we can all contribute meaningfully in controversial conversations with family, friends, neighbors, colleagues, and our representatives — and push for policies that protect those we care about. A little more than a hundred years ago, thousands of lives were routinely lost to preventive illnesses. We've come too far to let ignorance destroy us now.
A new type of cancer therapy is shrinking deadly brain tumors with just one treatment
MRI scans after a new kind of immunotherapy for brain cancer show remarkable progress in one patient just days after the first treatment.
Few cancers are deadlier than glioblastomas—aggressive and lethal tumors that originate in the brain or spinal cord. Five years after diagnosis, less than five percent of glioblastoma patients are still alive—and more often, glioblastoma patients live just 14 months on average after receiving a diagnosis.
But an ongoing clinical trial at Mass General Cancer Center is giving new hope to glioblastoma patients and their families. The trial, called INCIPIENT, is meant to evaluate the effects of a special type of immune cell, called CAR-T cells, on patients with recurrent glioblastoma.
How CAR-T cell therapy works
CAR-T cell therapy is a type of cancer treatment called immunotherapy, where doctors modify a patient’s own immune system specifically to find and destroy cancer cells. In CAR-T cell therapy, doctors extract the patient’s T-cells, which are immune system cells that help fight off disease—particularly cancer. These T-cells are harvested from the patient and then genetically modified in a lab to produce proteins on their surface called chimeric antigen receptors (thus becoming CAR-T cells), which makes them able to bind to a specific protein on the patient’s cancer cells. Once modified, these CAR-T cells are grown in the lab for several weeks so that they can multiply into an army of millions. When enough cells have been grown, these super-charged T-cells are infused back into the patient where they can then seek out cancer cells, bind to them, and destroy them. CAR-T cell therapies have been approved by the US Food and Drug Administration (FDA) to treat certain types of lymphomas and leukemias, as well as multiple myeloma, but haven’t been approved to treat glioblastomas—yet.
CAR-T cell therapies don’t always work against solid tumors, such as glioblastomas. Because solid tumors contain different kinds of cancer cells, some cells can evade the immune system’s detection even after CAR-T cell therapy, according to a press release from Massachusetts General Hospital. For the INCIPIENT trial, researchers modified the CAR-T cells even further in hopes of making them more effective against solid tumors. These second-generation CAR-T cells (called CARv3-TEAM-E T cells) contain special antibodies that attack EFGR, a protein expressed in the majority of glioblastoma tumors. Unlike other CAR-T cell therapies, these particular CAR-T cells were designed to be directly injected into the patient’s brain.
The INCIPIENT trial results
The INCIPIENT trial involved three patients who were enrolled in the study between March and July 2023. All three patients—a 72-year-old man, a 74-year-old man, and a 57-year-old woman—were treated with chemo and radiation and enrolled in the trial with CAR-T cells after their glioblastoma tumors came back.
The results, which were published earlier this year in the New England Journal of Medicine (NEJM), were called “rapid” and “dramatic” by doctors involved in the trial. After just a single infusion of the CAR-T cells, each patient experienced a significant reduction in their tumor sizes. Just two days after receiving the infusion, the glioblastoma tumor of the 72-year-old man decreased by nearly twenty percent. Just two months later the tumor had shrunk by an astonishing 60 percent, and the change was maintained for more than six months. The most dramatic result was in the 57-year-old female patient, whose tumor shrank nearly completely after just one infusion of the CAR-T cells.
The results of the INCIPIENT trial were unexpected and astonishing—but unfortunately, they were also temporary. For all three patients, the tumors eventually began to grow back regardless of the CAR-T cell infusions. According to the press release from MGH, the medical team is now considering treating each patient with multiple infusions or prefacing each treatment with chemotherapy to prolong the response.
While there is still “more to do,” says co-author of the study neuro-oncologist Dr. Elizabeth Gerstner, the results are still promising. If nothing else, these second-generation CAR-T cell infusions may someday be able to give patients more time than traditional treatments would allow.
“These results are exciting but they are also just the beginning,” says Dr. Marcela Maus, a doctor and professor of medicine at Mass General who was involved in the clinical trial. “They tell us that we are on the right track in pursuing a therapy that has the potential to change the outlook for this intractable disease.”
A recent study in The Lancet Oncology showed that AI found 20 percent more cancers on mammogram screens than radiologists alone.
Since the early 2000s, AI systems have eliminated more than 1.7 million jobs, and that number will only increase as AI improves. Some research estimates that by 2025, AI will eliminate more than 85 million jobs.
But for all the talk about job security, AI is also proving to be a powerful tool in healthcare—specifically, cancer detection. One recently published study has shown that, remarkably, artificial intelligence was able to detect 20 percent more cancers in imaging scans than radiologists alone.
Published in The Lancet Oncology, the study analyzed the scans of 80,000 Swedish women with a moderate hereditary risk of breast cancer who had undergone a mammogram between April 2021 and July 2022. Half of these scans were read by AI and then a radiologist to double-check the findings. The second group of scans was read by two researchers without the help of AI. (Currently, the standard of care across Europe is to have two radiologists analyze a scan before diagnosing a patient with breast cancer.)
The study showed that the AI group detected cancer in 6 out of every 1,000 scans, while the radiologists detected cancer in 5 per 1,000 scans. In other words, AI found 20 percent more cancers than the highly-trained radiologists.

But even though the AI was better able to pinpoint cancer on an image, it doesn’t mean radiologists will soon be out of a job. Dr. Laura Heacock, a breast radiologist at NYU, said in an interview with CNN that radiologists do much more than simply screening mammograms, and that even well-trained technology can make errors. “These tools work best when paired with highly-trained radiologists who make the final call on your mammogram. Think of it as a tool like a stethoscope for a cardiologist.”
AI is still an emerging technology, but more and more doctors are using them to detect different cancers. For example, researchers at MIT have developed a program called MIRAI, which looks at patterns in patient mammograms across a series of scans and uses an algorithm to model a patient's risk of developing breast cancer over time. The program was "trained" with more than 200,000 breast imaging scans from Massachusetts General Hospital and has been tested on over 100,000 women in different hospitals across the world. According to MIT, MIRAI "has been shown to be more accurate in predicting the risk for developing breast cancer in the short term (over a 3-year period) compared to traditional tools." It has also been able to detect breast cancer up to five years before a patient receives a diagnosis.
The challenges for cancer-detecting AI tools now is not just accuracy. AI tools are also being challenged to perform consistently well across different ages, races, and breast density profiles, particularly given the increased risks that different women face. For example, Black women are 42 percent more likely than white women to die from breast cancer, despite having nearly the same rates of breast cancer as white women. Recently, an FDA-approved AI device for screening breast cancer has come under fire for wrongly detecting cancer in Black patients significantly more often than white patients.
As AI technology improves, radiologists will be able to accurately scan a more diverse set of patients at a larger volume than ever before, potentially saving more lives than ever.

