An Electrifying Idea For Roads

Companies such as Wave and Magment offer a variety of approaches for charging vehicles without plugs while they're being stored or even used, but costs stand in the way of broader adoption.
Starting this summer, the public buses in the Oberhaching suburb of Munich, Germany, won’t have to be plugged in to charge overnight anymore. Stefan Schelle, the mayor of Oberhaching, is taking advantage of the fact that an innovative startup has its offices in his community: Magment, short for “magnetizing cement,” will install its underground charging pad in the coming months. As soon as that happens, the buses will charge while they wait at the city’s main station or while stored at their overnight quarters.
In his light-filled office, Magment’s co-founder and CEO, Mauricio Esguerra, demonstrates how the new technology works: The lights on his black model car only flash when he puts the miniature Porsche directly atop the induction plate. “This works just like when you charge your iPhone on its charging pad or heat a pot on an induction range. People don’t have to be afraid of magnetic fields or anything like that,” says the 60-year-old Colombia-born entrepreneur. “The induction only gets activated when the storage battery is placed directly on top.
Patented by Esguerra, the “magnetizing concrete” is able to target the charge quite precisely. The batteries will be mounted in a box underneath the vehicles such as the retrofitted public buses. “Look, here’s one passing by,” says Esguerra, pointing out the window as a blue city bus rides past his office.
An invention finds its purpose
Esguerra grew up in Bogotá, studied physics at the Technical University Munich where he fell in love with a German woman, and started a family in her home country. For 15 years, he developed magnetic products, including the magnetizing cement, for Siemens, Europe’s largest industrial manufacturing company. The patent belonged to Siemens, of course. “But there were hardly any electric vehicles yet,” Esguerra says, “and Siemens didn’t quite know what to do with this invention.”
Esguerra changed companies a few times but, in 2015, he got an offer from Siemens. The patent for the magnetizing cement was expiring and Siemens wasn’t interested in keeping it. Would he, as the inventor, want it back? “I did not hesitate a second,” Esguerra remembers with a smile. “I’m a magnetician at heart.” That same year, he founded Magment to finally make use of the technology he created 20 years ago.
To demonstrate how his cement is made, he opens the lid of a plastic bucket filled with cement powder. Mixed in are fingernail-sized black pieces, so-called ferrites, mainly consisting of three ceramic oxides: iron, nickel and zinc. Conventionally, they are used in electronics such as cell phones, computers and cables. Molded in concrete, ferrites create a magnetic field that can transport charge to a vehicle, potentially eliminating range anxiety for EV drivers.

Molded in concrete, ferrites create a magnetic field that can transport charge to a vehicle, potentially eliminating range anxiety for EV drivers.
Magment
“Ferrites have extremely high rejection rates,” Esguerra adds. “It’s comparable to other ceramics: As soon as there is a small tear or crack, the material is rejected. We are talking about a rejection pile of 500,000 tons per year worldwide. There are mountains of unused materials.”
Exactly this fact was the starting point of his research at Siemens: “What can we do with this energy-intensive material? Back then, it was crushed up and mixed into the cement for building streets, without adding any function.” Today, too, the Magment material can simply be mixed with the conventional material and equipment of the cement industry. “We take advantage of the fact that we don’t have to build factories and don’t have high transportation costs."
In addition to saving resources, recycled ferrite also makes concrete more durable.
No plugs, no charging breaks
A young intern in the office next door winds cables around a new coil. These coils will later be lowered underground in a box, connected to the grid and encased in magnetizing concrete. The recipient box looks similar; it’s another coil but smaller, and it will be mounted underneath the carriage of the vehicle. For a car, the battery box would be 25 by 25 centimeters (about 10 inches), for a scooter five by five centimeters (about two inches).
Esguerra pushes an electric scooter into a cemented scooter rack next to his office. The charging pad is invisible. A faint beep is the only sign that it has started charging. “Childs play!” Esguerra says. “Even when someone puts in the scooter a little crooked, the charge still works. Our efficiency rate is up to 96 percent.” From this summer on, hotel chains in Munich will try out this system with their rental scooters, at a price of about 500 Euros per charging station.
Compared to plug-in charging, Magment’s benefits include smaller batteries that charge slower and, therefore, gentler, so they may last longer. Nobody needs to plug in the vehicles manually anymore. “Personally, I’ve had an EV for six years,” Esguerra says, “and how often does it happen that I forgot to plug it in overnight and then start out with a low charge in the morning? Once people get used to the invisible charging system, it will become the norm.“
There are also downsides: Most car companies aren’t ready for the new technology. Hyundai is the first carmaker that announced plans to equip some new models with inductive charging capability. “How many cars are electrified worldwide?” Esguerra asks and gives the answer himself: “One percent. And how many forklifts are electrified? More than 70 percent!” Therefore, Magment focuses on charging forklifts, e-scooters and buses.

Magment has focused most of its efforts on charging forklifts and other vehicle types that are entirely or predominantly electric, unlike cars.
Magment
On the morning of my visit to Esguerra’s office, a developer of the world’s third-biggest forklift manufacturer is there to inspect how the technology works on the ground. In the basement, a Magment engineer drives an electric forklift over a testbed with invisible charging coils, turning on the green charging light. Esguerra opens the interior of the forklift and points out the two batteries. “With our system, the forklift will only need one battery.” The savings, about 7,000 Euro per forklift, will pay for the installation of Magment’s charging system in warehouses, Esguerra calculates. “Less personnel and no unnecessary wait times for charging will lead to further savings,” he says.
To implement the new technology as efficiently as possible, Magment engineers began recording the transport routes of forklifts in warehouses. “It looks like spaghetti diagrams,” Esguerra explains. “Soon you get the areas where the forklifts pass or wait most frequently. This is where you install the chargers underground.” The forklifts will charge while in use, without having to pause for charging breaks. The method could also work for robots, for instance, in warehouses and distribution centers.
Roads of the future could be electric
Potential disadvantages might become apparent once the technology is more broadly in use. Therefore investors were initially reluctant, Esguerra admits. “Some are eager to be the first but most prefer to wait until the technology has been extensively used in real life.”
A clear hurdle today is that electrifying entire freeways with induction coils would cost at least 1 to 1.5 million Euros per kilometer. The German Department for Transportation even calculates overall costs of 14 to 47 million Euros per kilometer. So, the technology may only make sense for areas where vehicles pass or dwell the longest, like the Oberhaching train station or a busy interstate toll booth.
And yet, Magment is ramping up to compete with other companies that build larger inductive charging pads. The company just finished the first 20 meters of a testbed in Indiana, in partnership with the Purdue University and the Indiana Department of Transportation. Magment is poised to build “the world’s first contactless wireless-charging concrete pavement highway segment,” Purdue University announced.
The project, part of Purdue’s ASPIRE (Advancing Sustainability through Powered Infrastructure for Roadway Electrification) program, is financed by the National Science Foundation. “Indiana is known as the Crossroads of America, and we’re committed to fortifying our position as a transportation leader by innovating to support the emerging vehicle technology,” Governor Eric J. Holcomb said. If testing is successful, including the concrete’s capacity to charge heavy trucks operating at higher power (200 kilowatts and above), Indiana plans to identify a highway segment to install Magment’s charging pads. The earliest would be 2023 at best.
In the meantime, buses in the Californian Antelope Valley, trams at Hollywood's Universal Studios and transit buses in Tampa, Florida, are already charging with inductive technology developed by Wave, a company spun out of Utah State University. In Michigan, Governor Gretchen Whitmer announced plans to build a test route for vehicles to charge while driving, in collaboration with the Israel-based company Electreon, and this year contracted to build the first road-based charging system in the U.S. The state is providing support through an innovative grant program.
Costs remain one of the biggest obstacles, but Esguerra’s vision includes the potential that toll roads could charge a premium for inductive charging capabilities. “And in reverse, a driver who has too much energy could feed his surplus into the grid while driving,” Esguerra dreams.
Meanwhile, Wave’s upcoming big projects are moving trucks along a route in Southern California and running a UPS route between Seattle and Portland. Wave CTO Michael Masquelier describes the inductive power transfer his company champions as “similar to a tuning fork. By vibrating that fork, you sent energy through the air and it is received by another tuning fork across the room. So it’s similar to that, but it’s magnetic energy versus sound energy.”
He hopes to partner with Magment, saying that “the magnetizing cement makes installation easier and improves the energy efficiency.” More research is needed to evaluate which company’s technology will prove to be the most efficient, practical, and cost-effective.
Esguerra’s vision includes the potential that toll roads could charge a premium for inductive charging capabilities. “And in reverse, a driver who has too much energy could feed his surplus into the grid while driving,” Esguerra dreams.
The future will soon arrive in the idyllic town of Bad Staffelstein, a quaint tourist destination in the Upper Franconia region of Germany. Visitors will be taken to and from the main station and the popular thermal bath by driverless shuttles. Together with the University of Wuppertal, the regional government of Upper Franconia wants to turn its district into “the center of autonomous driving.” Magment is about to install inductive charging pads at the shuttle stations and the thermal bath, eliminating the need for the shuttles to stop for charging times. No more drivers, no cable, no range anxiety. Masquelier believes that “wireless and autonomous driving go hand in hand.” Science fiction? It will become science reality in spring 2023.
CORRECTION: An earlier version of the story erroneously mentioned that Electreon required overhead cables.
Inside Scoop: How a DARPA Scientist Helped Usher in a Game-Changing Covid Treatment
Amy Jenkins is a program manager for the Defense Advanced Research Projects Agency's Biological Technologies Office, which runs a project called the Pandemic Prevention Platform.
Amy Jenkins was in her office at DARPA, a research and development agency within the Department of Defense, when she first heard about a respiratory illness plaguing the Chinese city of Wuhan. Because she's a program manager for DARPA's Biological Technologies Office, her colleagues started stopping by. "It's really unusual, isn't it?" they would say.
At the time, China had a few dozen cases of what we now call COVID-19. "We should maybe keep an eye on that," she thought.
Early in 2020, still just keeping watch, she was visiting researchers working on DARPA's Pandemic Prevention Platform (P3), a project to develop treatments for "any known or previously unknown infectious threat," within 60 days of its appearance. "We looked at each other and said, 'Should we be doing something?'" she says.
For projects like P3, groups of scientists—often at universities and private companies—compete for DARPA contracts, and program managers like Jenkins oversee the work. Those that won the P3 bid included scientists at AbCellera Biologics, Inc., AstraZeneca, Duke University, and Vanderbilt University.
At the time Jenkins was talking to the P3 performers, though, they didn't have evidence of community transmission. "We would have to cross that bar before we considered doing anything," she says.
The world soon leapt far over that bar. By the time Jenkins and her team decided P3 should be doing something—with their real work beginning in late February--it was too late to prevent this pandemic. But she could help P3 dig into the chemical foundations of COVID-19's malfeasance, and cut off its roots. That work represents, in fact, her roots.
In late February 2020, DARPA received a single blood sample from a recovered COVID-19 patient, in which P3 researchers could go fishing for antibodies. The day it arrived, Jenkins's stomach roiled. "We get one shot," she thought.
Fighting the Smallest Enemies
Jenkins, who's in her early 40s, first got into germs the way many 90s kids did: by reading The Hot Zone, a novel about a hemorrhagic fever gone rogue. It wasn't exactly the disintegrating organs that hooked her. It was the idea that "these very pathogens that we can't even see can make us so sick and bring us to our knees," she says. Reading about scientists facing down deadly disease, she wondered, "How do these things make you so sick?"
She chased that question in college, majoring in both biomolecular science and chemistry, and later became an antibody expert. Antibodies are proteins that hook to a pathogen to block it from attaching to your cells, or tag it for destruction by the rest of the immune system. Soon, she jumped on the "monoclonal antibodies" train—developing synthetic versions of these natural defenses, which doctors can give to people to help them battle an early-stage infection, and even to prevent an infection from taking root after an exposure.
Jenkins likens the antibody treatments to the old aphorism about fishing: Vaccines teach your body how to fish, but antibodies simply give your body the pesca-fare. While that, as the saying goes, won't feed you for a lifetime, it will last a few weeks or months. Monoclonal antibodies thus are a promising preventative option in the immediate short-term when a vaccine hasn't yet been given (or hasn't had time to produce an immune response), as well as an important treatment weapon in the current fight. After former president Donald Trump contracted COVID-19, he received a monoclonal antibody treatment from biotech company Regeneron.
As for Jenkins, she started working as a DARPA Biological Technologies Office contractor soon after completing her postdoc. But it was a suit job, not a labcoat job. And suit jobs, at first, left Jenkins conflicted, worried about being bored. She'd give it a year, she thought. But the year expired, and bored she was not. Around five years later, in June 2019, the agency hired her to manage several of the office's programs. A year into that gig, the world was months into a pandemic.
The Pandemic Pivot
At DARPA, Jenkins inherited five programs, including P3. P3 works by taking blood from recovered people, fishing out their antibodies, identifying the most effective ones, and then figuring out how to manufacture them fast. Back then, P3 existed to help with nebulous, future outbreaks: Pandemic X. Not this pandemic. "I did not have a crystal ball," she says, "but I will say that all of us in the infectious diseases and public-health realm knew that the next pandemic was coming."
Three days after a January 2020 meeting with P3 researchers, COVID-19 appeared in Seattle, then began whipping through communities. The time had come for P3 teams to swivel. "We had done this," she says. "We had practiced this before." But would their methods stand up to something unknown, racing through the global population? "The big anxiety was, 'Wow, this was real,'" says Jenkins.
While facing down that realness, Jenkins was also managing other projects. In one called PREPARE, groups develop "medical countermeasures" that modulate a person's genetic code to boost their bodies' responses to threats. Another project, NOW, envisions shipping-container-sized factories that can make thousands of vaccine doses in days. And then there's Prometheus—which means "forethought" in Greek, and is the name of the god who stole fire and gave it to humans. Wrapping up as COVID ramped up, Prometheus aimed to identify people who are contagious—with whatever—before they start coughing, and even if they never do.
All of DARPA's projects focus on developing early-stage technology, passing it off to other agencies or industry to put it into operation. The orientation toward a specific goal appealed to Jenkins, as a contrast to academia. "You go down a rabbit hole for years at a time sometimes, chasing some concept you found interesting in the lab," she says. That's good for the human pursuit of knowledge, and leads to later applications, but DARPA wants a practical prototype—stat.
"Dual-Use" Technologies
That desire, though, and the fact that DARPA is a defense agency, present philosophical complications. "Bioethics in the national-security context turns all the dials up to 10+," says Jonathan Moreno, a medical ethicist at the University of Pennsylvania.
While developing antibody treatments to stem a pandemic seems straightforwardly good, all biological research—especially that backed by military money—requires evaluating potential knock-on applications, even those that might come from outside the entity that did the developing. As Moreno put it, "Albert Einstein wasn't thinking about blowing up Hiroshima." Particularly sensitive are so-called "dual-use" technologies—those tools that could be used for both benign and nefarious purposes, or are of interest to both the civilian and military worlds.
Moreno takes Prometheus itself as an example of "dual-use" technology. "Think about somebody wearing a suicide vest. Instead of a suicide vest, make them extremely contagious with something. The flu plus Ebola," he says. "Send them someplace, a sensitive environment. We would like to be able to defend against that"—not just tell whether Uncle Fred is bringing asymptomatic COVID home for Christmas. Prometheus, Jenkins says, had safety in mind from the get-go, and required contenders to "develop a risk mitigation plan" and "detail their strategy for appropriate control of information."
To look at a different program, if you can modulate genes to help healing, you probably know something (or know someone else could infer something) about how to hinder healing. Those sorts of risks are why PREPARE researchers got their own "ethical, legal, and social implications" panel, which meets quarterly "to ensure that we are performing all research and publications in a safe and ethical manner," says Jenkins.
DARPA as a whole, Moreno says, is institutionally sensitive to bioethics. The agency has ethics panels, and funded a 2014 National Academies assessment of how to address the "ethical, legal, and societal issues" around technology that has military relevance. "In the cases of biotechnologies where some of that research brushes up against what could legitimately be considered dual-use, that in itself justifies our investment," says Jenkins. "DARPA deliberately focuses on safety and countermeasures against potentially dangerous technologies, and we structure our programs to be transparent, safe, and legal."
Going Fishing
In late February 2020, DARPA received a single blood sample from a recovered COVID-19 patient, in which P3 researchers could go fishing for antibodies. The day it arrived, Jenkins's stomach roiled. "We get one shot," she thought.
As scientists from the P3-funded AbCellera went through the processes they'd practiced, Jenkins managed their work, tracking progress and relaying results. Soon, the team had isolated a suitable protein: bamlanivimab. It attaches to and blocks off the infamous spike proteins on SARS-CoV-2—those sticky suction-cups in illustrations. Partnering with Eli Lilly in a manufacturing agreement, the biotech company brought it to clinical trials in May, just a few months after its work on the deadly pathogen began, after much of the planet became a hot zone.
On November 10—Jenkins's favorite day at the (home) office—the FDA provided Eli Lilly emergency use authorization for bamlanivimab. But she's only mutedly screaming (with joy) inside her heart. "This pandemic isn't 'one morning we're going to wake up and it's all over,'" she says. When it is over, she and her colleagues plan to celebrate their promethean work. "I'm hoping to be able to do it in person," she says. "Until then, I have not taken a breath."
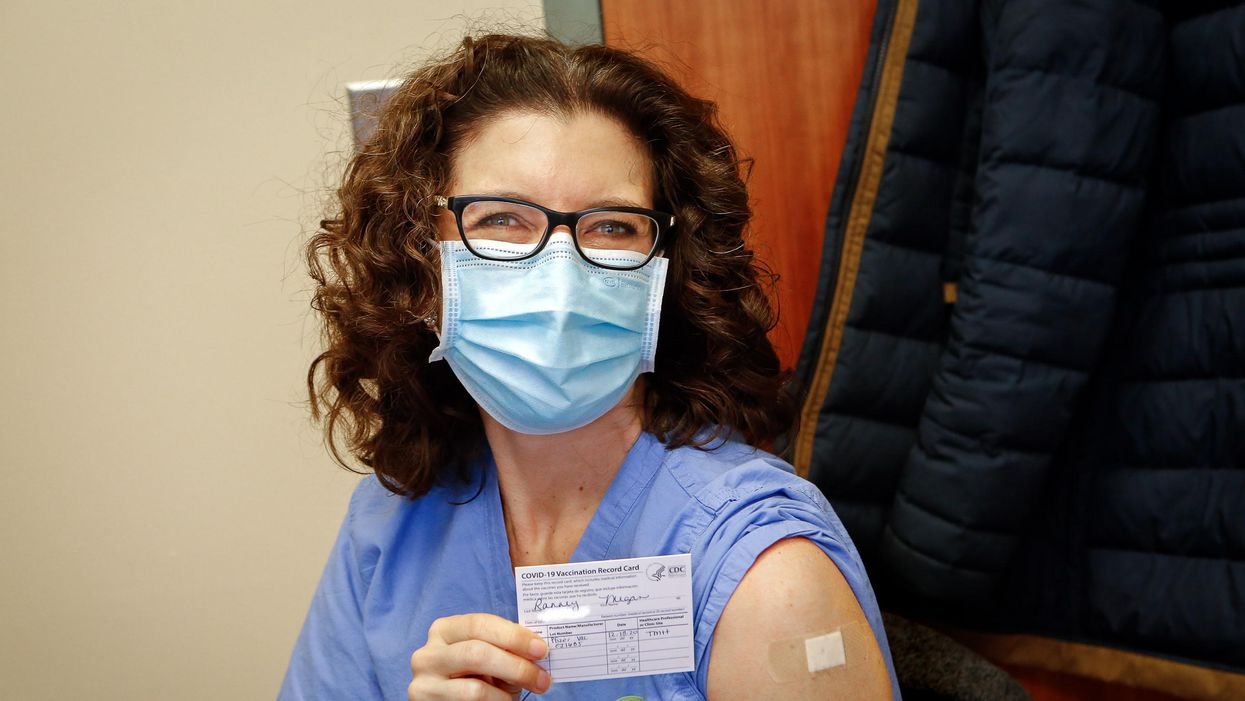
Everyone Should Hear My COVID Vaccine Experience
Dr. Ranney immediately after receiving her first dose of the Pfizer vaccine on December 18, 2020.
On December 18th, 2020, I received my first dose of the Pfizer mRNA vaccine against SARS-CoV-2. On January 9th, 2021, I received my second. I am now a CDC-card-carrying, fully vaccinated person.
The build-up to the first dose was momentous. I was scheduled for the first dose of the morning. Our vaccine clinic was abuzz with excitement and hope, and some media folks were there to capture the moment. A couple of fellow emergency physicians were in the same cohort of recipients as I; we exchanged virtual high-fives and took a picture of socially distanced hugs. It was, after all, the closest thing we'd had to a celebration in months.
I walked in the vaccine administration room with anticipation – it was tough to believe this moment was truly, finally here. I got a little video of my getting the shot, took my obligate vaccine selfie, waited in the observation area for 15 minutes to ensure I didn't have a reaction, and then proudly joined 1000s of fellow healthcare workers across the country in posting #ThisIsMyShot on social media. "Here we go, America!"
The first shot, though, didn't actually do all that much for me. It hurt less than a flu shot (which, by the way, doesn't hurt much). I had virtually no side effects. I also knew that it did not yet protect me. The Pfizer (and Moderna) data show very clearly that although the immune response starts to grow 10-12 days after the first shot, one doesn't reach full protection against COVID-19 until much later.
So when, two days after my first shot, I headed back to work in the emergency department, I kept wondering "Will this be the day that I get sick? Wouldn't that be ironic!" Although I never go without an N95 during patient care, it just takes one slip – scratching one's eyes, eating lunch in a break room that an infected colleague had just been in – to get ill. Ten months into this pandemic, it is so easy to get fatigued, to make a small error just one time.
Indeed, I had a few colleagues fall ill in between their first and second shots; one was hospitalized. This was not surprising, but still sad, given how close they had come to escaping infection.
Scientifically speaking, one doesn't need to feel bad to develop an immune response. Emotionally, though, I welcomed the symptoms as proof positive that I would be protected.
This time period felt a little like we had our learner's permit for driving: we were on our way to being safe, but not quite there yet.
I also watched, with dismay, our failures as a nation at timely distribution of the vaccine. On December 18th, despite the logistical snafus that many of us had started to highlight, it was still somewhat believable that we would at least distribute (if not actually administer) 20 million doses by the New Year. But by December 31, my worst fears about the feds' lack of planning had been realized. Only 14 million doses had gone to states, and fewer than 3 million had been administered. Within the public health and medical community, we began to debate how to handle the shortages and slow vaccination rates: should we change prioritization schemes? Get rid of the second dose, in contradiction to what our FDA had approved?
Let me be clear: I really, really, really wanted my second dose. It is what is supported by the data. After living this long at risk, it felt frankly unfair that I might not get fully protected. I waited with trepidation, afraid that policies would shift before I got it in my arm.
At last, my date for my second shot arrived.
This shot was a little less momentous on the outside. The vaccine clinic was much more crowded, as we were now administering first doses to more people, as well as providing the second dose to many. There were no high fives, no media, and I took no selfies. I finished my observation period without trouble (as did everyone else vaccinated the same day, as is typical for these vaccines). I walked out the door planning to spend a nice afternoon outdoors with my kids.
Within 15 minutes, though, the very common side effects – reported by 80% of people my age after the second dose – began to appear. First I got a headache (like 52% of people my age), then body aches (37%), fatigue (59%), and chills (35%). I felt "foggy", like I was fighting something. Like 45% of trial participants who had received the actual vaccine, I took acetaminophen and ibuprofen to stave off the symptoms. There is some minimal evidence from other vaccines that pre-treatment with these anti-inflammatories may reduce antibodies, but given that half of trial participants took these medications, there's no reason to make yourself suffer if you develop side effects. Forty-eight hours later, just in time for my next shift, the side effects magically cleared. Scientifically speaking, one doesn't need to feel bad to develop an immune response. Emotionally, though, I welcomed the symptoms as proof positive that I would be protected.
My reaction was truly typical. Although the media hype focuses on major negative reactions, they are – statistically speaking – tremendously rare: fewer than 11/million people who received the Pfizer vaccine, and 3/million who received the Moderna vaccine, developed anaphylaxis; of these, all were treated, and all are fine. Compare this with the fact that approximately 1200/million Americans have died of this virus. I'll choose the minor, temporary, utterly treatable side effects any day.
Now, more than 14 days after my second dose, the data says that my chance of getting really sick is, truly, infinitesimally low. I don't have to worry that each shift will put me into the hospital. I feel emotionally lighter, and a little bit like I have a secret super-power.
But I also know that we are not yet home free.
I may have my personal equivalent of Harry Potter's invisibility cloak – but we don't yet know whether it protects those around me, at all. As Dr. Fauci himself has written, while community spread is high, there is still a chance that I could be a carrier of infection to others. So I still wear my N95 at work, I still mask in public, and I still shower as soon as I get home from a shift and put my scrubs right in the washing machine to protect my husband and children. I also won't see my parents indoors until they, too, have been vaccinated.
At the end of the day, these vaccines are both amazing and life-changing, and not. My colleagues are getting sick less often, now that many of us are a week or more out from our second dose. I can do things (albeit still masked) that would simply not have been safe a month ago. These are small miracles, for which I am thankful. But like so many things in life, they would be better if shared with others. Only when my community is mostly vaccinated, will I breathe easy again.
My deepest hope is that we all have – and take - the chance to get our shots, soon. Because although the symbolism and effect of the vaccine is high, the experience itself was … not that big a deal.