A skin patch to treat peanut allergies teaches the body to tolerate the nuts

Peanut allergies affect about a million children in the U.S., and most never outgrow them. Luckily, some promising remedies are in the works.
Ever since he was a baby, Sharon Wong’s son Brandon suffered from rashes, prolonged respiratory issues and vomiting. In 2006, as a young child, he was diagnosed with a severe peanut allergy.
"My son had a history of reacting to traces of peanuts in the air or in food,” says Wong, a food allergy advocate who runs a blog focusing on nut free recipes, cooking techniques and food allergy awareness. “Any participation in school activities, social events, or travel with his peanut allergy required a lot of preparation.”
Peanut allergies affect around a million children in the U.S. Most never outgrow the condition. The problem occurs when the immune system mistakenly views the proteins in peanuts as a threat and releases chemicals to counteract it. This can lead to digestive problems, hives and shortness of breath. For some, like Wong’s son, even exposure to trace amounts of peanuts could be life threatening. They go into anaphylactic shock and need to take a shot of adrenaline as soon as possible.
Typically, people with peanut allergies try to completely avoid them and carry an adrenaline autoinjector like an EpiPen in case of emergencies. This constant vigilance is very stressful, particularly for parents with young children.
“The search for a peanut allergy ‘cure’ has been a vigorous one,” says Claudia Gray, a pediatrician and allergist at Vincent Pallotti Hospital in Cape Town, South Africa. The closest thing to a solution so far, she says, is the process of desensitization, which exposes the patient to gradually increasing doses of peanut allergen to build up a tolerance. The most common type of desensitization is oral immunotherapy, where patients ingest small quantities of peanut powder. It has been effective but there is a risk of anaphylaxis since it involves swallowing the allergen.
"By the end of the trial, my son tolerated approximately 1.5 peanuts," Sharon Wong says.
DBV Technologies, a company based in Montrouge, France has created a skin patch to address this problem. The Viaskin Patch contains a much lower amount of peanut allergen than oral immunotherapy and delivers it through the skin to slowly increase tolerance. This decreases the risk of anaphylaxis.
Wong heard about the peanut patch and wanted her son to take part in an early phase 2 trial for 4-to-11-year-olds.
“We felt that participating in DBV’s peanut patch trial would give him the best chance at desensitization or at least increase his tolerance from a speck of peanut to a peanut,” Wong says. “The daily routine was quite simple, remove the old patch and then apply a new one. By the end of the trial, he tolerated approximately 1.5 peanuts.”
How it works
For DBV Technologies, it all began when pediatric gastroenterologist Pierre-Henri Benhamou teamed up with fellow professor of gastroenterology Christopher Dupont and his brother, engineer Bertrand Dupont. Together they created a more effective skin patch to detect when babies have allergies to cow's milk. Then they realized that the patch could actually be used to treat allergies by promoting tolerance. They decided to focus on peanut allergies first as the more dangerous.
The Viaskin patch utilizes the fact that the skin can promote tolerance to external stimuli. The skin is the body’s first defense. Controlling the extent of the immune response is crucial for the skin. So it has defense mechanisms against external stimuli and can promote tolerance.
The patch consists of an adhesive foam ring with a plastic film on top. A small amount of peanut protein is placed in the center. The adhesive ring is attached to the back of the patient's body. The peanut protein sits above the skin but does not directly touch it. As the patient sweats, water droplets on the inside of the film dissolve the peanut protein, which is then absorbed into the skin.
The peanut protein is then captured by skin cells called Langerhans cells. They play an important role in getting the immune system to tolerate certain external stimuli. Langerhans cells take the peanut protein to lymph nodes which activate T regulatory cells. T regulatory cells suppress the allergic response.
A different patch is applied to the skin every day to increase tolerance. It’s both easy to use and convenient.
“The DBV approach uses much smaller amounts than oral immunotherapy and works through the skin significantly reducing the risk of allergic reactions,” says Edwin H. Kim, the division chief of Pediatric Allergy and Immunology at the University of North Carolina, U.S., and one of the principal investigators of Viaskin’s clinical trials. “By not going through the mouth, the patch also avoids the taste and texture issues. Finally, the ability to apply a patch and immediately go about your day may be very attractive to very busy patients and families.”
Brandon Wong displaying origami figures he folded at an Origami Convention in 2022
Sharon Wong
Clinical trials
Results from DBV's phase 3 trial in children ages 1 to 3 show its potential. For a positive result, patients who could not tolerate 10 milligrams or less of peanut protein had to be able to manage 300 mg or more after 12 months. Toddlers who could already tolerate more than 10 mg needed to be able to manage 1000 mg or more. In the end, 67 percent of subjects using the Viaskin patch met the target as compared to 33 percent of patients taking the placebo dose.
“The Viaskin peanut patch has been studied in several clinical trials to date with promising results,” says Suzanne M. Barshow, assistant professor of medicine in allergy and asthma research at Stanford University School of Medicine in the U.S. “The data shows that it is safe and well-tolerated. Compared to oral immunotherapy, treatment with the patch results in fewer side effects but appears to be less effective in achieving desensitization.”
The primary reason the patch is less potent is that oral immunotherapy uses a larger amount of the allergen. Additionally, absorption of the peanut protein into the skin could be erratic.
Gray also highlights that there is some tradeoff between risk and efficacy.
“The peanut patch is an exciting advance but not as effective as the oral route,” Gray says. “For those patients who are very sensitive to orally ingested peanut in oral immunotherapy or have an aversion to oral peanut, it has a use. So, essentially, the form of immunotherapy will have to be tailored to each patient.” Having different forms such as the Viaskin patch which is applied to the skin or pills that patients can swallow or dissolve under the tongue is helpful.
The hope is that the patch’s efficacy will increase over time. The team is currently running a follow-up trial, where the same patients continue using the patch.
“It is a very important study to show whether the benefit achieved after 12 months on the patch stays stable or hopefully continues to grow with longer duration,” says Kim, who is an investigator in this follow-up trial.
"My son now attends university in Massachusetts, lives on-campus, and eats dorm food. He has so much more freedom," Wong says.
The team is further ahead in the phase 3 follow-up trial for 4-to-11-year-olds. The initial phase 3 trial was not as successful as the trial for kids between one and three. The patch enabled patients to tolerate more peanuts but there was not a significant enough difference compared to the placebo group to be definitive. The follow-up trial showed greater potency. It suggests that the longer patients are on the patch, the stronger its effects.
They’re also testing if making the patch bigger, changing the shape and extending the minimum time it’s worn can improve its benefits in a trial for a new group of 4-to-11 year-olds.
The future
DBV Technologies is using the skin patch to treat cow’s milk allergies in children ages 1 to 17. They’re currently in phase 2 trials.
As for the peanut allergy trials in toddlers, the hope is to see more efficacy soon.
For Wong’s son who took part in the earlier phase 2 trial for 4-to-11-year-olds, the patch has transformed his life.
“My son continues to maintain his peanut tolerance and is not affected by peanut dust in the air or cross-contact,” Wong says. ”He attends university in Massachusetts, lives on-campus, and eats dorm food. He still carries an EpiPen but has so much more freedom than before his clinical trial. We will always be grateful.”
Three Big Biotech Ideas to Watch in 2020—And Beyond
Body-on-a-chip, prime editing, and gut microbes all are poised to make a big impact in 2020.
1. Happening Now: Body-on-a-Chip Technology Is Enabling Safer Drug Trials and Better Cancer Research
Researchers have increasingly used the technology known as "lab-on-a-chip" or "organ-on-a-chip" to test the effects of pharmaceuticals, toxins, and chemicals on humans. Rather than testing on animals, which raises ethical concerns and can sometimes be inaccurate, and human-based clinical trials, which can be expensive and difficult to iterate, scientists turn to tiny, micro-engineered chips—about the size of a thumb drive.
It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip.
The chips are lined with living samples of human cells, which mimic the physiology and mechanical forces experienced by cells inside the human body, down to blood flow and breathing motions; the functions of organs ranging from kidneys and lungs to skin, eyes, and the blood-brain barrier.
A more recent—and potentially even more useful—development takes organ-on-a-chip technology to the next level by integrating several chips into a "body-on-a-chip." Since human organs don't work in isolation, seeing how they all react—and interact—once a foreign element has been introduced can be crucial to understanding how a certain treatment will or won't perform. Dr. Shyni Varghese, a MEDx investigator at the Duke University School of Medicine, is one of the researchers working with these systems in order to gain a more nuanced understanding of how multiple different organs react to the same stimuli.
Her lab is working on "tumor-on-a-chip" models, which can not only show the progression and treatment of cancer, but also model how other organs would react to immunotherapy and other drugs. "The effect of drugs on different organs can be tested to identify potential side effects," Varghese says. In addition, these models can help the researchers figure out how cancers grow and spread, as well as how to effectively encourage immune cells to move in and attack a tumor.
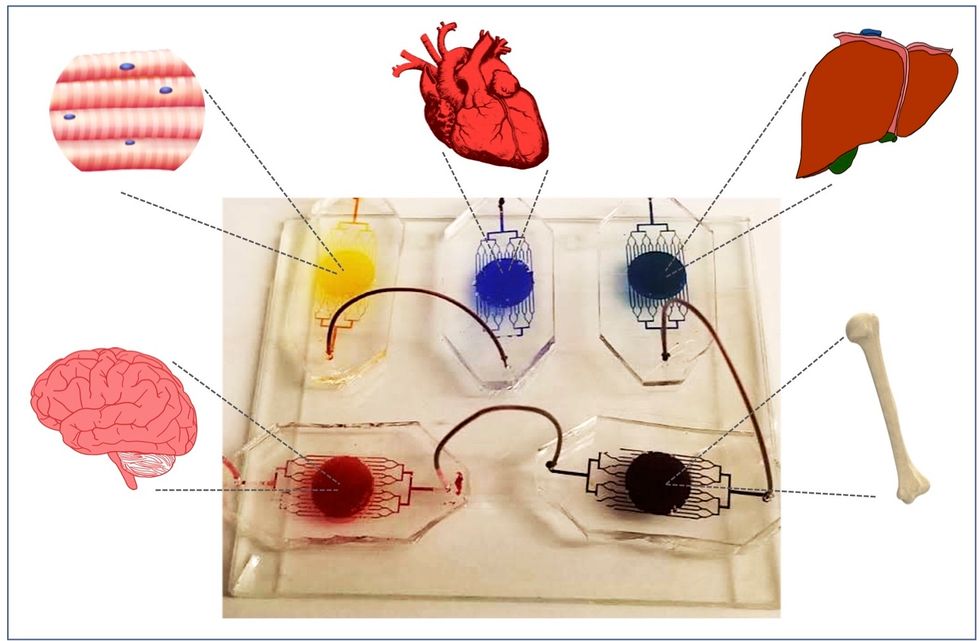
One body-on-a-chip used by Dr. Varghese's lab tracks the interactions of five organs—brain, heart, liver, muscle, and bone.
As their research progresses, Varghese and her team are looking for ways to maintain the long-term function of the engineered organs. In addition, she notes that this kind of research is not just useful for generalized testing; "organ-on-chip technologies allow patient-specific analyses, which can be used towards a fundamental understanding of disease progression," Varghese says. It's possible that doctors could one day take individual cell samples and create personalized treatments, testing out any medications on the chip for safety, efficacy, and potential side effects before writing a prescription.
2. Happening Soon: Prime Editing Will Have the Power to "Find and Replace" Disease-Causing Genes
Biochemist David Liu made industry-wide news last fall when he and his lab at MIT's Broad Institute, led by Andrew Anzalone, published a paper on prime editing: a new, more focused technology for editing genes. Prime editing is a descendant of the CRISPR-Cas9 system that researchers have been working with for years, and a cousin to Liu's previous innovation—base editing, which can make a limited number of changes to a single DNA letter at a time.
By contrast, prime editing has the potential to make much larger insertions and deletions; it also doesn't require the tweaked cells to divide in order to write the changes into the DNA, which could make it especially suitable for central nervous system diseases, like Parkinson's.
Crucially, the prime editing technique has a much higher efficiency rate than the older CRISPR system, and a much lower incidence of accidental insertions or deletions, which can make dangerous changes for a patient.
It also has a very broad potential range: according to Liu, 89% of the pathogenic mutations that have been collected in ClinVar (a public archive of human variations) could, in principle, be treated with prime editing—although he is careful to note that correcting a single genetic mutation may not be sufficient to fully treat a genetic disease.
Figuring out just how prime editing can be used most effectively and safely will be a long process, but it's already underway. The same day that Liu and his team posted their paper, they also made the basic prime editing constructs available for researchers around the world through Addgene, a plasmid repository, so that others in the scientific community can test out the technique for themselves. It might be years before human patients will see the results, and in the meantime, significant bioethical questions remain about the limits and sociological effects of such a powerful gene-editing tool. But in the long fight against genetic diseases, it's a huge step forward.
3. Happening When We Fund It: Focusing on Microbiome Health Could Help Us Tackle Social Inequality—And Vice Versa
The past decade has seen a growing awareness of the major role that the microbiome, the microbes present in our digestive tract, play in human health. Having a less-healthy microbiome is correlated with health risks like diabetes and depression, and interventions that target gut health, ranging from kombucha to fecal transplants, have cropped up with increasing frequency.
New research from the University of Maine's Dr. Suzanne Ishaq takes an even broader view, arguing that low-income and disadvantaged populations are less likely to have healthy, diverse gut bacteria, and that increasing access to beneficial microorganisms is an important juncture of social justice and public health.
"Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
"Typically, having a more diverse bacterial community is associated with health, and having fewer different species is associated with illness and may leave you open to infection from bacteria that are good at exploiting opportunities," Ishaq says.
Having a healthy biome doesn't mean meeting one fixed ratio of gut bacteria, since different combinations of microbes can generate roughly similar results when they work in concert. Generally, "good" microbes are the ones that break down fiber and create the byproducts that we use for energy, or ones like lactic acid bacteria that work to make microbials and keep other bacteria in check. The microbial universe in your gut is chaotic, Ishaq says. "Microbes in your gut interact with each other, with you, with your food, or maybe they don't interact at all and pass right through you." Overall, it's tricky to name specific microbial communities that will make or break someone's health.
There are important corollaries between environment and biome health, though, which Ishaq points out: Living in urban environments reduces microbial exposure, and losing the microorganisms that humans typically source from soil and plants can reduce our adaptive immunity and ability to fight off conditions like allergies and asthma. Access to green space within cities can counteract those effects, but in the U.S. that access varies along income, education, and racial lines. Likewise, lower-income communities are more likely to live in food deserts or areas where the cheapest, most convenient food options are monotonous and low in fiber, further reducing microbial diversity.
Ishaq also suggests other areas that would benefit from further study, like the correlation between paid family leave, breastfeeding, and gut microbiota. There are technical and ethical challenges to direct experimentation with human populations—but that's not what Ishaq sees as the main impediment to future research.
"The biggest roadblock is money, and the solution is also money," she says. "Basically, allowing people to lead healthy lives allows them to access and recruit microbes."
That means investment in things we already understand to improve public health, like better education and healthcare, green space, and nutritious food. It also means funding ambitious, interdisciplinary research that will investigate the connections between urban infrastructure, housing policy, social equity, and the millions of microbes keeping us company day in and day out.
Scientists Just Started Testing a New Class of Drugs to Slow--and Even Reverse--Aging
Eliminating "zombie-like" cells, called senescent cells, may hold the key to slowing aging and its chronic diseases.
Imagine reversing the processes of aging. It's an age-old quest, and now a study from the Mayo Clinic may be the first ray of light in the dawn of that new era.
The immune system can handle a certain amount of senescence, but that capacity declines with age.
The small preliminary report, just nine patients, primarily looked at the safety and tolerability of the compounds used. But it also showed that a new class of small molecules called senolytics, which has proven to reverse markers of aging in animal studies, can work in humans.
Aging is a relentless assault of chronic diseases including Alzheimer's, cardiovascular disease, diabetes, and frailty. Developing one chronic condition strongly predicts the rapid onset of another. They pile on top of each other and impede the body's ability to respond to the next challenge.
"Potentially, by targeting fundamental aging processes, it may be possible to delay or prevent or alleviate multiple age-related conditions and many diseases as a group, instead of one at a time," says James Kirkland, the Mayo Clinic physician who led the study and is a top researcher in the growing field of geroscience, the biology of aging.
Getting Rid of "Zombie" Cells
One element common to many of the diseases is senescence, a kind of limbo or zombie-like state where cells no longer divide or perform many regular functions, but they don't die. Senescence is thought to be beneficial in that it inhibits the cancerous proliferation of cells. But in aging, the senescent cells still produce molecules that create inflammation both locally and throughout the body. It is a cycle that feeds upon itself, slowly ratcheting down normal body function and health.
Disease and harmful stimuli like radiation to treat cancer can also generate senescence, which is why young cancer patients seem to experience earlier and more rapid aging. The immune system can handle a certain amount of senescence, but that capacity declines with age. There also appears to be a threshold effect, a tipping point where senescence becomes a dominant factor in aging.
Kirkland's team used an artificial intelligence approach called machine learning to look for cell signaling networks that keep senescent cells from dying. To date, researchers have identified at least eight such signaling networks, some of which seem to be unique to a particular type of cell or tissue, but others are shared or overlap.
Then a computer search identified molecules known to disrupt these signaling pathways "and allow cells that are fully senescent to kill themselves," he explains. The process is a bit like looking for the right weapons in a video game to wipe out lingering zombie cells. But instead of swords, guns, and grenades, the list of biological tools so far includes experimental molecules, approved drugs, and natural supplements.
Treatment
"We found early on that targeting single components of those networks will only kill a very small minority of senescent cells or senescent cell types," says Kirkland. "So instead of going after one drug-one target-one disease, we're going after networks with combinations of drugs or drugs that have multiple targets. And we're going after every age-related disease."
The FDA is grappling with guidance for researchers wanting to conduct clinical trials on something as broad as aging rather than a single disease.
The large number of potential senolytic (i.e. zombie-neutralizing) compounds they identified allowed Kirkland to be choosy, "purposefully selecting drugs where the side effects profile was good...and with short elimination half-lives." The hit and run approach meant they didn't have to worry about maintaining a steady state of drugs in the body for an extended period of time. Some of the compounds they selected need only a half hour exposure to trigger the dying process in senescent cells, which can then take several days.
Work in mice has already shown impressive results in reversing diabetes, weight gain, Alzheimer's, cardiovascular disease and other conditions using senolytic agents.
That led to Kirkland's pilot study in humans with diabetes-related kidney disease using a three-day regimen of dasatinib, a kinase inhibitor first approved in 2006 to treat some forms of blood cancer, and quercetin, a flavonoid found in many plants and sold as a food supplement.
The combination was safe and well tolerated; it reduced the number of senescent cells in the belly fat of patients and restored their normal function, according to results published in September in the journal EBioMedicine. This preliminary paper was based on 9 patients in an ongoing study of 30 patients.
Kirkland cautions that these are initial and incomplete findings looking primarily at safety issues, not effectiveness. There is still much to be learned about the use of senolytics, starting with proof that they actually provide clinical benefit, and against what chronic conditions. The drug combinations, doses, duration, and frequency, not to mention potential risks all must be worked out. Additional studies of other diseases are being developed.
What's Next
Ron Kohanski, a senior administrator at the NIH National Institute on Aging (NIA), says the field of senolytics is so new that there isn't even a consensus on how to identify a senescent cell, and the FDA is grappling with guidance for researchers wanting to conduct clinical trials on something as broad as aging rather than a single disease.
Intellectual property concerns may temper the pharmaceutical industry's interest in developing senolytics to treat chronic diseases of aging. It looks like many mix-and-match combinations are possible, and many of the potential molecules identified so far are found in nature or are drugs whose patents have or will soon expire. So the ability to set high prices for such future drugs, and hence the willingness to spend money on expensive clinical trials, may be limited.
Still, Kohanski believes the field can move forward quickly because it often will include products that are already widely used and have a known safety profile. And approaches like Kirkland's hit and run strategy will minimize potential exposure and risk.
He says the NIA is going to support a number of clinical trials using these new approaches. Pharmaceutical companies may feel that they can develop a unique part of a senolytic combination regimen that will justify their investment. And if they don't, countries with socialized medicine may take the lead in supporting such research with the goal of reducing the costs of treating aging patients.

