Food Poisoning Outbreaks Are Still A Problem. Powerful Tech Is Fighting Back.

The latest tools, like whole genome sequencing, are allowing food outbreaks to be identified and stopped more quickly.
With the pandemic at the forefront of everyone's minds, many people have wondered if food could be a source of coronavirus transmission. Luckily, that "seems unlikely," according to the CDC, but foodborne illnesses do still sicken a whopping 48 million people per year.
Whole genome sequencing is like "going from an eight-bit image—maybe like what you would see in Minecraft—to a high definition image."
In normal times, when there isn't a historic global health crisis infecting millions and affecting the lives of billions, foodborne outbreaks are real and frightening, potentially deadly, and can cause widespread fear of particular foods. Think of Romaine lettuce spreading E. coli last year— an outbreak that infected more than 500 people and killed eight—or peanut butter spreading salmonella in 2008, which infected 167 people.
The technologies available to detect and prevent the next foodborne disease outbreak have improved greatly over the past 30-plus years, particularly during the past decade, and better, more nimble technologies are being developed, according to experts in government, academia, and private industry. The key to advancing detection of harmful foodborne pathogens, they say, is increasing speed and portability of detection, and the precision of that detection.
Getting to Rapid Results
Researchers at Purdue University have recently developed a lateral flow assay that, with the help of a laser, can detect toxins and pathogenic E. coli. Lateral flow assays are cheap and easy to use; a good example is a home pregnancy test. You place a liquid or liquefied sample on a piece of paper designed to detect a single substance and soon after you get the results in the form of a colored line: yes or no.
"They're a great portable tool for us for food contaminant detection," says Carmen Gondhalekar, a fifth-year biomedical engineering graduate student at Purdue. "But one of the areas where paper-based lateral flow assays could use improvement is in multiplexing capability and their sensitivity."
J. Paul Robinson, a professor in Purdue's Colleges of Veterinary Medicine and Engineering, and Gondhalekar's advisor, agrees. "One of the fundamental problems that we have in detection is that it is hard to identify pathogens in complex samples," he says.
When it comes to foodborne disease outbreaks, you don't always know what substance you're looking for, so an assay made to detect only a single substance isn't always effective. The goal of the project at Purdue is to make assays that can detect multiple substances at once.
These assays would be more complex than a pregnancy test. As detailed in Gondhalekar's recent paper, a laser pulse helps create a spectral signal from the sample on the assay paper, and the spectral signal is then used to determine if any unique wavelengths associated with one of several toxins or pathogens are present in the sample. Though the handheld technology has yet to be built, the idea is that the results would be given on the spot. So someone in the field trying to track the source of a Salmonella infection could, for instance, put a suspected lettuce sample on the assay and see if it has the pathogen on it.
"What our technology is designed to do is to give you a rapid assessment of the sample," says Robinson. "The goal here is speed."
Seeing the Pathogen in "High-Def"
"One in six Americans will get a foodborne illness every year," according to Dr. Heather Carleton, a microbiologist at the Centers for Disease Control and Prevention's Enteric Diseases Laboratory Branch. But not every foodborne outbreak makes the news. In 2017 alone, the CDC monitored between 18 and 37 foodborne poison clusters per week and investigated 200 multi-state clusters. Hardboiled eggs, ground beef, chopped salad kits, raw oysters, frozen tuna, and pre-cut melon are just a taste of the foods that were investigated last year for different strains of listeria, salmonella, and E. coli.
At the heart of the CDC investigations is PulseNet, a national network of laboratories that uses DNA fingerprinting to detect outbreaks at local and regional levels. This is how it works: When a patient gets sick—with symptoms like vomiting and fever, for instance—they will go to a hospital or clinic for treatment. Since we're talking about foodborne illnesses, a clinician will likely take a stool sample from the patient and send it off to a laboratory to see if there is a foodborne pathogen, like salmonella, E. Coli, or another one. If it does contain a potentially harmful pathogen, then a bacterial isolate of that identified sample is sent to a regional public health lab so that whole genome sequencing can be performed.
Whole genome sequencing can differentiate "virtually all" strains of foodborne pathogens, no matter the species, according to the FDA.
Whole genome sequencing is a method for reading the entire genome of a bacterial isolate (or from any organism, for that matter). Instead of working with a couple dozen data points, now you're working with millions of base pairs. Carleton likes to describe it as "going from an eight-bit image—maybe like what you would see in Minecraft—to a high definition image," she says. "It's really an evolution of how we detect foodborne illnesses and identify outbreaks."
If the bacterial isolate matches another in the CDC's database, this means there could be a potential outbreak and an investigation may be started, with the goal of tracking the pathogen to its source.
Whole genome sequencing has been a relatively recent shift in foodborne disease detection. For more than 20 years, the standard technique for analyzing pathogens in foodborne disease outbreaks was pulsed-field gel electrophoresis. This method creates a DNA fingerprint for each sample in the form of a pattern of about 15-30 "bands," with each band representing a piece of DNA. Researchers like Carleton can use this fingerprint to see if two samples are from the same bacteria. The problem is that 15-30 bands are not enough to differentiate all isolates. Some isolates whose bands look very similar may actually come from different sources and some whose bands look different may be from the same source. But if you can see the entire DNA fingerprint, then you don't have that issue. That's where whole genome sequencing comes in.
Although the PulseNet team had piloted whole genome sequencing as early as 2013, it wasn't until July of last year that the transition to using whole genome sequencing for all pathogens was complete. Though whole genome sequencing requires far more computing power to generate, analyze, and compare those millions of data points, the payoff is huge.
Stopping Outbreaks Sooner
The U.S. Food and Drug Administration (FDA) acquired their first whole genome sequencers in 2008, according to Dr. Eric Brown, the Director of the Division of Microbiology in the FDA's Office of Regulatory Science. Since then, through their GenomeTrakr program, a network of more than 60 domestic and international labs, the FDA has sequenced and publicly shared more than 400,000 isolates. "The impact of what whole genome sequencing could do to resolve a foodborne outbreak event was no less impactful than when NASA turned on the Hubble Telescope for the first time," says Brown.
Whole genome sequencing has helped identify strains of Salmonella that prior methods were unable to differentiate. In fact, whole genome sequencing can differentiate "virtually all" strains of foodborne pathogens, no matter the species, according to the FDA. This means it takes fewer clinical cases—fewer sick people—to detect and end an outbreak.
And perhaps the largest benefit of whole genome sequencing is that these detailed sequences—the millions of base pairs—can imply geographic location. The genomic information of bacterial strains can be different depending on the area of the country, helping these public health agencies eventually track the source of outbreaks—a restaurant, a farm, a food-processing center.
Coming Soon: "Lab in a Backpack"
Now that whole genome sequencing has become the go-to technology of choice for analyzing foodborne pathogens, the next step is making the process nimbler and more portable. Putting "the lab in a backpack," as Brown says.
The CDC's Carleton agrees. "Right now, the sequencer we use is a fairly big box that weighs about 60 pounds," she says. "We can't take it into the field."
A company called Oxford Nanopore Technologies is developing handheld sequencers. Their devices are meant to "enable the sequencing of anything by anyone anywhere," according to Dan Turner, the VP of Applications at Oxford Nanopore.
"The sooner that we can see linkages…the sooner the FDA gets in action to mitigate the problem and put in some kind of preventative control."
"Right now, sequencing is very much something that is done by people in white coats in laboratories that are set up for that purpose," says Turner. Oxford Nanopore would like to create a new, democratized paradigm.
The FDA is currently testing these types of portable sequencers. "We're very excited about it. We've done some pilots, to be able to do that sequencing in the field. To actually do it at a pond, at a river, at a canal. To do it on site right there," says Brown. "This, of course, is huge because it means we can have real-time sequencing capability to stay in step with an actual laboratory investigation in the field."
"The timeliness of this information is critical," says Marc Allard, a senior biomedical research officer and Brown's colleague at the FDA. "The sooner that we can see linkages…the sooner the FDA gets in action to mitigate the problem and put in some kind of preventative control."
At the moment, the world is rightly focused on COVID-19. But as the danger of one virus subsides, it's only a matter of time before another pathogen strikes. Hopefully, with new and advancing technology like whole genome sequencing, we can stop the next deadly outbreak before it really gets going.
These doctors have a heart for recycling
In the U.S. and Europe, it is illegal to reuse pacemakers and other implants. Therefore, cardiologists export them to the global South where they save the lives of people of all ages.
This is part 3 of a three part series on a new generation of doctors leading the charge to make the health care industry more sustainable - for the benefit of their patients and the planet. Read part 1 here and part 2 here.
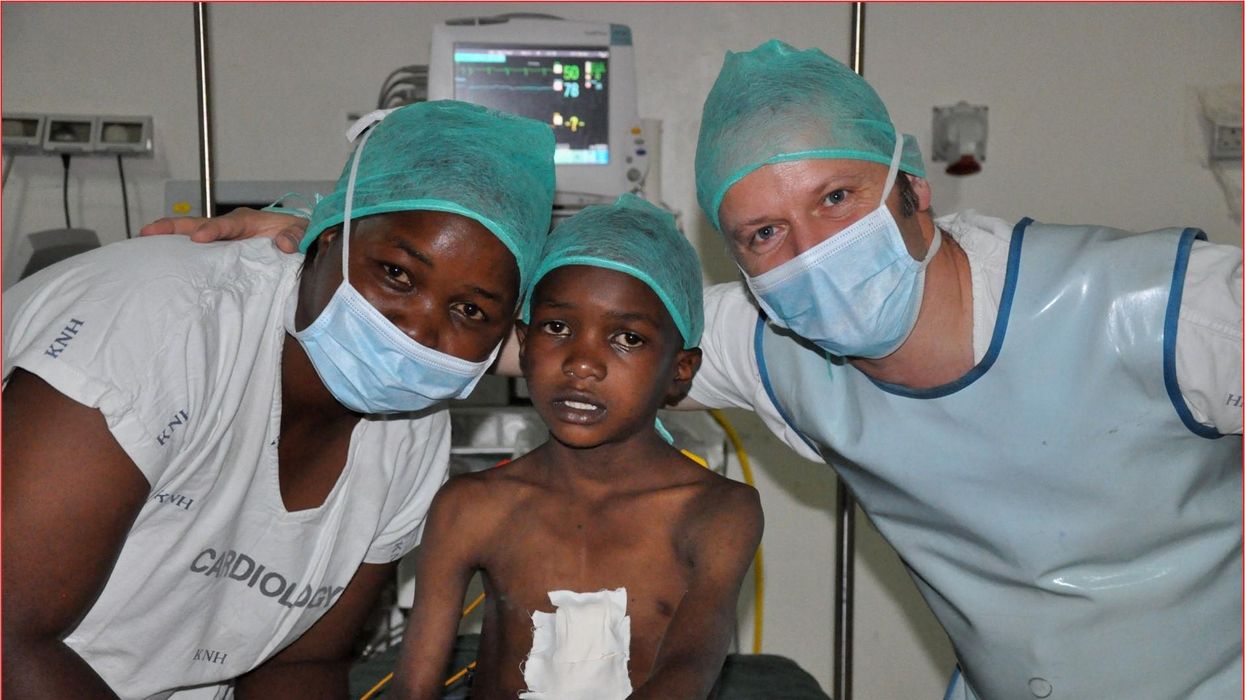
One could say that over 400 people owe their life to the fact that Carsten Israel fell in love. Twenty years ago, as a young doctor in Frankfurt, Germany, he began to court an au pair from Kenya, Elisabeth, his now-wife of 13 years with whom he has three children. When the couple started visiting her parents in Kenya, Israel wanted to check out the local hospitals, “just out of professional curiosity,“ says the cardiologist, who is currently the head doctor at the Clinic for Interior Medicine in Bielefeld. “I was completely shocked.“
Often he observed there were no doctors in the E.R.s, and hte nurses could render only basic first aid. “When somebody fell into a coma, they fell into a coma,“ Israel remembers. “There weren’t even any defibrillators to restart a patient’s heart,” while defibrillators are standard equipment in most clinics in the U.S. and Europe as lifesaving devices. When Israel finally visited the largest and most modern hospital in Nairobi, he found it better equipped but he learned that its services were only available to patients who could afford them. The cardiologist there had a drawer full of petitions from patients with heart ailments who couldn’t afford lifesaving surgery. Even two decades ago, a pacemaker cost $5,000 in Kenya, which made it unaffordable for most Kenyans who earn an average of $600 per month.
Since 2003, Israel and a team of two doctors and two nurses visit Kenya and Zambia once or twice a year to implant German pacemakers for free. Notably, the pacemakers and defibrillators Israel exports to Africa would end up in the landfill in Germany. Clinics have to pay for specialized services to dispose of this medical equipment. “In Germany, I could go to jail if I used a defibrillator that is one day past its expiration date,“ Israel says, “but in Kenya, people don’t have the money for the cheapest model. What nonsense to throw this precious medical equipment away while people in poorer countries die because they desperately need it.“
Israel works at the breakpoint between the laws in a wealthy country like Germany and the reality in the global South. The U.S. and most European countries have strict laws that ban the reuse of medical implants and enforce strict expiration dates for medical equipment. “But if a pacemaker is a few days past its expiration date, it still works perfectly fine,“ Israel says. “And it also happens that we implant a pacemaker and five months later it turns out that the patient needs a different kind. Then we replace it and we’d have to trash the first one in Germany, though it could easily run another 12 years.“
“If we get this right, we have lots of devices we can implant, hips and knees, etcetera. Where this will lead is limitless," says Eva Kline Rogers, the program coordinator for My Heart, Your Heart.
Israel has been collecting donations of pacemakers and defibrillators from manufacturers but also from other doctors and from funeral homes for his nonprofit Pacemakers for East Africa since 2003. Most funeral homes in the U.S. and Europe are legally obliged to remove pacemakers from the dead before cremation. “Most pacemakers survive their owners,“ says Israel. He sterilizes the pacemakers and finds them a new life in East Africa. Studies show that reused pacemakers carry no greater risk for the patients than new ones.
In the U.S., University of Michigan professor Thomas Crawford heads up a similar initiative, My Heart, Your Heart. “Each year 1 to 2 million individuals worldwide die due to a lack of access to pacemakers and defibrillators,” the organization notes on its website. The nonprofit was founded in 2009, but it took four years for the doctors to get permission from the FDA to export pacemakers. Since receiving permission, the organization has sent dozens of devices to the Philippines, Haiti, Venezuela, Kenya, Sierra Leone and Ukraine. “We were the first doctors ever to implant a pacemaker in Sierra Leone in 2018,” says Crawford, who has traveled extensively to most of the recipient countries.
Even individuals can donate their pacemakers; the organization offers a prepaid envelope. “My mother recently passed and she donated her device,” says Tina Alexandris-Souphis, one of the doctors at University of Michigan who collaborates on My Heart, Your Heart. The organization works with World Medical Relief and the U.K. based charity Pace4Life to maintain a registry of the most urgent patients and send devices to where they are needed the most.
My Heart, Your Heart is also conducting a randomized controlled trial to provide further evidence that reused pacemakers pose no additional risk. “Our vision is that we establish this is safe and create a blueprint for organizations around the world to safely reuse these devices instead of them being thrown in the trash,” says Eva Kline Rogers, the program’s coordinator. “If we get this right, we have lots of devices we can implant, hips and knees, etc. Where this will lead is limitless.” She points out that in addition to receiving the donated devices, the doctors in the global South also benefit from the expertise of renowned cardiologists, such as Crawford, who sometimes advise them in complex cases.
And Adrian Baranchuk, a Canadian doctor at the Kingston General Hospital at the Queen’s University, regularly travels through South America with his “cardiology van” to help villagers in remote areas with heart problems.
Israel says that he’s been accused of racism, in thinking that these pacemakers are suitable for those in the global South - many of whom are people of color - even though officials in wealthier countries consider them to be trash. The cardiologist counters such criticism with stories about desperate need of his patients. At his first medical visit to Nairobi that he organized with a local cardiologist, six patients were waiting for him. “In Germany, they would all be considered emergencies,” Israel says. One eighty-year old grandmother had a heartrate of 18. “I’ve never before seen anything like this,” Israel exclaims. “At first I thought I couldn’t find her pulse before I realized that her heart was only beating once every three seconds.” After the surgery, she got up, dressed herself and hurriedly packed her bag, explaining she had a ton of work to accomplish. Her family was in disbelief, Israel says. “They told me she had been bedridden for five years because as soon as she tried to get up she would faint.”

Israel has been accused of racism, in thinking that these pacemakers are suitable for those in the global South even though they're considered to be trash by officials in wealthier countries. The cardiologist counters such criticism with stories about desperate need of his patients.
Carsten Israel
The hospital in Nairobi where Israel conducts the surgeries, charges patients $200 for the use of its facilities. If patients can’t afford that sum, Israel will pay it from the funds of his nonprofit. For some people, $200 far exceeds their resources. Once, a family from the extremely poor Northern region of Kenya told him they couldn’t afford the $3 for the bus ride to Nairobi. Israel suspected this was a pretense because they were afraid of the surgery and agreed to reimburse the $3, “but when they came, they were wearing rags and were so rail-thin, I understood that they really needed every cent they had for food.”
Israel is a renowned cardiologists in Germany. And yet, he considers his work in East Africa to be particularly meaningful. “Generally, most patients in Germany will get the treatment they need,” he says, “and I never before experienced that people have an illness that is easily curable but simply won’t be treated.” He also feels a heavy responsibility. Many patients have his personal cell phone and call him when they have problems or good news about how they’re doing.
Some of those progress reports come much faster than in Israel’s home country. Before he implanted a pacemaker in a tall Massai in Kenya, the man had been picked on by his family because he wouldn’t help much with the hard work on the family peanut farm. “When I examined him, he had a pulse of 40,” Israel says. “It’s a miracle he was even standing upright, let alone hauling heavy bags.” After the surgery, Israel advised his patient to stay the night for observation, but the patient couldn’t wait to leave. Two hours later, he returned, covered in sweat. He’d been running sprints with his brothers to test the new device. Israel shakes his head. In Germany, it would be unthinkable for a patient to engage in athletics immediately after surgery. But the patient was exuberant: “I was the fastest!”
The success stories are notable partly because the challenges remain so steep. In Zambia, for instance, there is a single cardiologist; she determined to become one after losing her younger sister to an easily curable heart disease. Often, the hospitals not only lack pacemakers but also sterile surgery equipment, antibiotics and other essential material. Therefore, Israel and his team import everything they need for the surgeries, including medication. If necessary, they improvise. “I’ve done surgery with a desk lamp hanging from the ceiling by threads,” Israel says. He already knows that he will need to return to Kenya in six months to replace the pacemaker of one of his patients and replace the batteries in others. If he doesn’t travel, lives are at risk.These technologies may help more animals and plants survive climate change
As the climate changes, the ripples will reach everywhere. Better data is needed for both plants and animals, and scientists are looking for genes that could allow crops to survive.
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are making us more vulnerable to infectious diseases by land and by sea - and how scientists are working on solutions.
Along the west coast of South Florida and the Keys, Florida Bay is a nursery for young Caribbean spiny lobsters, a favorite local delicacy. Growing up in small shallow basins, they are especially vulnerable to warmer, more saline water. Climate change has brought tidal floods, bleached coral reefs and toxic algal blooms to the state, and since the 1990s, the population of the Caribbean spiny lobster has dropped some 20 percent, diminishing an important food for snapper, grouper, and herons, as well as people. In 1999, marine ecologist Donald Behringer discovered the first known virus among lobsters, Panulirus argus virus—about a quarter of juveniles die from it before they mature.
“When the water is warm PaV1 progresses much more quickly,” says Behringer, who is based at the Emerging Pathogens Institute at the University of Florida in Gainesville.
Caribbean spiny lobsters are only one example of many species that are struggling in the era of climate change, both at sea and on land. As the oceans heat up, absorbing greenhouse gases and growing more acidic, marine diseases are emerging at an accelerated rate. Marine creatures are migrating to new places, and carrying pathogens with them. The latest grim report in the journal Science, states that if global warming continues at the current rate, the extinction of marine species will rival the Permian–Triassic extinction, sometimes called the “Great Dying,” when volcanoes poisoned the air and wiped out as much as 90 percent of all marine life 252 million years ago.
Similarly, on land, climate change has exposed wildlife, trees and crops to new or more virulent pathogens. Warming environments allow fungi, bacteria, viruses and infectious worms to proliferate in new species and locations or become more virulent. One paper modeling records of nearly 1,400 wildlife species projects that parasites will double by 2070 in the far north and in high-altitude places. Right now, we are seeing the effects most clearly on the fringes—along the coasts, up north and high in the mountains—but as the climate continues changing, the ripples will reach everywhere.
Few species are spared
On the Hawaiian Islands, mosquitoes are killing more songbirds. The dusky gray akikiki of Kauai and the chartreuse-yellow kiwikiu of Maui could vanish in two years, under assault from mosquitoes bearing avian malaria, according to a University of Hawaiʻi 2022 report. Previously, the birds could escape infection by roosting high in the cold mountains, where the pests couldn’t thrive, but climate change expanded the range of the mosquito and narrowed theirs.
Likewise, as more midge larvae survive over warm winters and breed better during drier summers, they bite more white-tailed deer, spreading often-fatal epizootic hemorrhagic disease. Especially in northern regions of the globe, climate change brings the threat of midges carrying blue tongue disease, a virus, to sheep and other animals. Tick-borne diseases like encephalitis and Lyme disease may become a greater threat to animals and perhaps humans.
"If you put all your eggs in one basket and then a pest comes a long, then you are more vulnerable to those risks," says Mehroad Ehsani, managing director of the food initiative in Africa for the Rockefeller Foundation. "Research is needed on resilient, climate smart, regenerative agriculture."
In the “thermal mismatch” theory of wildlife disease, cold-adapted species are at greater risk when their habitats warm, and warm-adapted species suffer when their habitats cool. Mammals can adjust their body temperature to adapt to some extent. Amphibians, fish and insects that cannot regulate body temperatures may be at greater risk. Many scientists see amphibians, especially, as canaries in the coalmine, signaling toxicity.
Early melting ice can foster disease. Climate models predict that the spring thaw will come ever-earlier in the lakes of the French Pyrenees, for instance, which traditionally stayed frozen for up to half the year. The tadpoles of the midwife toad live under the ice, where they are often infected with amphibian chytrid fungus. When a seven-year study tracked the virus in three species of amphibians in Pyrenees’s Lac Arlet, the research team found that, the earlier the spring thaw arrived, the more infection rates rose in common toads— , while remaining high among the midwife toads. But the team made another sad discovery: with early thaws, the common frog, which was thought to be free of the disease in Europe, also became infected with the fungus and died in large numbers.
Changing habitats affect animal behavior. Normally, spiny lobsters rely on chemical cues to avoid predators and sick lobsters. New conditions may be hampering their ability to “social distance”—which may help PaV1 spread, Behringer’s research suggests. Migration brings other risks. In April 2022, an international team led by scientists at Georgetown University announced the first comprehensive overview, published in the journal Nature, of how wild mammals under pressure from a changing climate may mingle with new populations and species—giving viruses a deadly opportunity to jump between hosts. Droughts, for example, will push animals to congregate at the few places where water remains.
Plants face threats also. At the timberline of the cold, windy, snowy mountains of the U.S. west, whitebark pine forests are facing a double threat, from white pine blister rust, a fungal disease, and multiplying pine beetles. “If we do nothing, we will lose the species,” says Robert Keane, a research ecologist for the U.S. Forest Service, based in Missoula, Montana. That would be a huge shift, he explains: “It’s a keystone species. There are over 110 animals that depend on it, many insects, and hundreds of plants.” In the past, beetle larvae would take two years to complete their lifecycle, and many died in frost. “With climate change, we're seeing more and more beetles survive, and sometimes the beetle can complete its lifecycle in one year,” he says.
Quintessential crops are under threat too
As some pathogens move north and new ones develop, they pose novel threats to the crops humans depend upon. This is already happening to wheat, coffee, bananas and maize.
Breeding against wheat stem rust, a fungus long linked to famine, was a key success in the mid-20th century Green Revolution, which brought higher yields around the world. In 2013, wheat stem rust reemerged in Germany after decades of absence. It ravaged both bread and durum wheat in Sicily in 2016 and has spread as far as England and Ireland. Wheat blast disease, caused by a different fungus, appeared in Bangladesh in 2016, and spread to India, the world’s second largest producer of wheat.
Insects, moths, worms, and coffee leaf rust—a fungus now found in all coffee-growing countries—threaten the livelihoods of millions of people who grow coffee, as well as everybody’s cup of joe. More heat, more intense rain, and higher humidity have allowed coffee leaf rust to cycle more rapidly. It has grown exponentially, overcoming the agricultural chemicals that once kept it under control.
To identify new diseases and fine-tune crops for resistance, scientists are increasingly relying on genomic tools.
Tar spot, a fungus native to Latin America that can cut corn production in half, has emerged in highland areas of Central Mexico and parts of the U.S.. Meanwhile, maize lethal necrosis disease has spread to multiple countries in Africa, notes Mehrdad Ehsani, Managing Director for the Food Initiative in Africa of the Rockefeller Foundation. The Cavendish banana, which most people eat today, was bred to be resistant to the fungus Panama 1. Now a new fungus, Panama 4, has emerged on every continent–including areas of Latin America that rely on the Cavendish for their income, reported a recent story in the Guardian. New threats are poised to emerge. Potato growers in the Andes Mountains have been shielded from disease because of colder weather at high altitude, but temperature fluxes and warming weather are expected to make this crop vulnerable to potato blight, found plant pathologist Erica Goss, at the Emerging Pathogens Institute.
Science seeks solutions
To protect food supplies in the era of climate change, scientists are calling for integrated global surveillance systems for crop disease outbreaks. “You can imagine that a new crop variety that is drought-tolerant could be susceptible to a pathogen that previous varieties had some resistance against,” Goss says. “Or a country suffers from a calamitous weather event, has to import seed from another country, and that seed is contaminated with a new pathogen or more virulent strain of an existing pathogen.” Researchers at the John Innes Center in Norwich and Aarhus University in Denmark have established ways to monitor wheat rust, for example.
Better data is essential, for both plants and animals. Historically, models of climate change predicted effects on plant pathogens based on mean temperatures, and scientists tracked plant responses to constant temperatures, explains Goss. “There is a need for more realistic tests of the effects of changing temperatures, particularly changes in daily high and low temperatures on pathogens,” she says.
To identify new diseases and fine-tune crops for resistance, scientists are increasingly relying on genomic tools. Goss suggests factoring the impact of climate change into those tools. Genomic efforts to select soft red winter wheat that is resistant to Fusarium head blight (FHB), a fungus that plagues farmers in the Southeastern U.S., have had early success. But temperature changes introduce a new factor.
A fundamental solution would be to bring back diversification in farming, says Ehsani. Thousands of plant species are edible, yet we rely on a handful. Wild relatives of domesticated crops are a store of possibly useful genes that may confer resistance to disease. The same is true for livestock. “If you put all your eggs in one basket and then a pest comes along, then you are more vulnerable to those risks. Research is needed on resilient, climate smart, regenerative agriculture,” Ehsani says.
Jonathan Sleeman, director of the U.S. Geological Survey National Wildlife Health Center, has called for data on wildlife health to be systematically collected and integrated with climate and other variables because more comprehensive data will result in better preventive action. “We have focused on detecting diseases,” he says, but a more holistic strategy would apply human public health concepts to assuring animal wellbeing. (For example, one study asked experts to draw a diagram of relationships of all the factors affecting the health of a particular group of caribou.) We must not take the health of plants and animals for granted, because their vulnerability inevitably affects us too, Sleeman says. “We need to improve the resilience of wildlife populations so they can withstand the impact of climate change.”