More Families Are Using Nanny Cams to Watch Elderly Loved Ones, Raising Ethical Questions

Jackie Costanzo and her 93-year-old mom, Louise, are happy to have an extra way to stay connected with the camera, which is normally placed on a television stand facing her mom's bed.
After Jackie Costanzo's mother broke her right hip in a fall, she needed more hands-on care in her assisted-living apartment near Sacramento, California. A social worker from her health plan suggested installing a video camera to help ensure those services were provided.
Without the camera, Costanzo wouldn't have a way to confirm that caregivers had followed through with serving meals, changing clothes, and fulfilling other care needs.
When Costanzo placed the device in May 2018, she informed the administrator and staff, and at first, there were no objections. The facility posted a sign on the apartment's front door, alerting anyone who entered of recording in progress.
But this past spring, a new management company came across the sign and threatened to issue a 30-day eviction notice to her 93-year-old mother, Louise Munch, who has dementia, for violating a policy that prohibits cameras in residents' rooms. With encouragement from California Advocates for Nursing Home Reform, Costanzo researched the state's regulations but couldn't find anything to support or deny camera use. She refused to remove the recording device and prevailed.
"In essence, my mom was 'grandfathered in' because she moved in under a management company that did not specify that residents could not have cameras," says Costanzo, 73, a retired elementary schoolteacher who lives a three-hour drive away, in Silicon Valley, and visits one day every two weeks. Without the camera, Costanzo, who is her mother's only surviving child, wouldn't have a way to confirm that caregivers had followed through with serving meals, changing clothes, and fulfilling other care needs.
As technological innovations enable next of kin to remain apprised of the elderly's daily care in long-term care facilities, surveillance cameras bring legal and privacy issues to the forefront of a complex ethical debate. Families place them overtly or covertly—disguised in a makeshift clock radio, for instance—when they suspect or fear abuse or neglect, so they can maintain a watchful eye, perhaps deterring egregious behavior. But the cameras also capture intimate caregiving tasks, such as bathing and toileting, as well as dressing and undressing, which may undermine the dignity of residents.
So far, laws or guidelines in eight states—Illinois, Maryland, New Mexico, Oklahoma, Texas, Utah, Virginia, and Washington—have granted families the rights to install cameras in a resident's room. In addition, about 15 other states have proposed legislation. Some states, such as Pennsylvania, have put forth regulatory compliance guidance, according to a column published in the July/August 2018 issue of Annals of Long-Term Care.
The increasing prevalence of this legislation has placed it on the radar of long-term care providers. It also suggests a trend to clarify responsible camera use in monitoring services while respecting privacy, says Victor Lane Rose, the column's editor and director of aging services at ECRI Institute, a health care nonprofit near Philadelphia, Pennsylvania.
In most cases, a resident's family installs a camera or instigates a request in hopes of sparing their loved one from the harms of abuse, says James Wright, a family physician who serves as the ethics committee's vice chair of the Society for Post-Acute and Long-Term Care Medicine in Columbia, Maryland. A camera also allows the family to check in on the resident from afar and remain on alert for a potential fall or agitated state, he says.
"It's rare that a facility will have 24-hour presence in a patient's room. You won't have a nurse in there all the time," says Wright, who is also medical director of two long-term care centers and one assisted-living facility around Richmond, Virginia. Particularly "with dementia, the family often wonders" if their loved one is safe.
While offering families peace of mind, he notes that video cameras can also help exonerate caregivers accused of abuse or theft. Hearing aids, which typically cost between $2,000 and $3,000 each, often go missing. By reviewing a video together, families and administrators may find clues to a device's disappearance. Conversely, Wright empathizes with the main counterargument against camera use, which is the belief that "invasion of privacy is also invasion of human dignity."
In respecting modesty, ethical questions abound over whether a camera should be turned off when a patient is in the midst of receiving personal care, such as dressing and undressing or using bedpans. Other ethical issues revolve around who may access the recordings, says Lori Smetanka, executive director of the National Consumer Voice for Quality Long-Term Care in Washington, D.C.
Video cameras, she contends, are only one tool in shielding residents from abuse. They are "not substitutes for personal involvement," she says. "People need to be very vigilant visiting their family members, and facilities have a responsibility to ensure that residents are free of abuse."
Lack of accountability perpetuates abuse in long-term care settings and stems in large part from systemic underfunding.
Educating employees in abuse prevention becomes paramount, and families should ask about staff training before placing their loved one in a long-term care facility, Smetanka says. Prior to installing a camera, she recommends consulting an attorney who is familiar with this issue.
But thoughts of a camera often don't occur to families until an adverse event affects their loved one, says Toby Edelman, a senior policy attorney at the Center for Medicare Advocacy, a nonprofit organization with headquarters in Washington, D.C., and Connecticut.
"These cameras can show exactly what's going on," she explains, noting that prosecutors have used the recordings in litigation. "When residents have injuries of unknown origin" and they can't verbalize what happened to them, "the cameras may document that yes, the resident was actually hit by somebody."
With a resident's safety and security being "the most important consideration," the American Health Care Association in Washington, D.C., which represents long-term and post-acute care providers, supports allowing states, clinicians, and patients to decide about camera use on a local level, says David Gifford, senior vice president of quality and regulatory affairs and chief medical officer.
"We've seen some success with tools such as permissive legislation, where residents and their loved ones have the ability to determine whether a camera is right for them while working with the center openly and ensuring the confidentiality of other residents," says Gifford, who practiced as a geriatrician. "It is important to note, however, that surveillance cameras are still only one element of the quality matrix. We can never hope to truly improve quality care by catching bad actors after the fact."
Lack of accountability perpetuates abuse in long-term care settings and stems in large part from systemic underfunding. Low wages and morale are tied to high turnover, and cameras don't address this overarching problem, says Clara Berridge, an assistant professor of social work at the University of Washington in Seattle, who has co-authored articles on surveillance devices in elder care.
Employees often don't perceive a nursing assistant position as a long-term career trajectory and may not feel vested in the workplace. Training in the recognition and reporting of abuse becomes ineffective when workers quit shortly thereafter. Many must juggle multiple jobs to make ends meet. Staffing shortages are endemic, leading to inadequate oversight of residents and voicing of abuse complaints, she says.
In Berridge's assessment, cameras may do more harm than good. Respondents to a survey she conducted of nursing homes and assisted-living facilities in the United States found that recording devices tend to fuel workers' anxiety amid a culture that further demoralizes and dehumanizes the care they provide.
Consent becomes particularly thorny in shared rooms, which are more common than not in nursing homes. States that permit in-room cameras mandate that roommates or their legal representative be made aware. Even if the camera is directed away from their bed, it will still capture conversations as well as movements that enter its scope. "Surveillance isn't the best way to protect adults in need of support," Berridge says. "Public investment in quality care is."
"The camera is invaluable. But there's no law that says you can have it automatically, so that's wrong."
In the one-bedroom assisted-living apartment where Costanzo's mother lives alone, consent from another resident wasn't needed. Without a roommate, the camera is much less intrusive, although Costanzo wishes she had put one in the living room, not just the bedroom, for more security.
Her safety concerns escalated when she read about a Texas serial killer who smothered victims after gaining access to senior care facilities by "masquerading as a maintenance man." She points to such horrifying incidents, although exceedingly rare, as further justification for permitting cameras to help guard the vulnerable against abuse in long-term care settings. And she hopes to advocate for an applicable law in California.
"The camera is invaluable," says Costanzo, who pays for monthly Wi-Fi service so she can see and interact with her mother, who turns 94 in October, any time of day or night. "But there's no law that says you can have it automatically, so that's wrong."
Meet Dr. Renee Wegrzyn, the first Director of President Biden's new health agency, ARPA-H
Today's podcast guest, Dr. Renee Wegrzyn, directs ARPA-H, a new agency formed last year to spearhead health innovations. Time will tell if ARPA-H will produce advances on the level of its fellow agency, DARPA.
In today’s podcast episode, I talk with Renee Wegrzyn, appointed by President Biden as the first director of a health agency created last year, the Advanced Research Projects Agency for Health, or ARPA-H. It’s inspired by DARPA, the agency that develops innovations for the Defense department and has been credited with hatching world-changing technologies such as ARPANET, which became the internet.
Time will tell if ARPA-H will lead to similar achievements in the realm of health. That’s what President Biden and Congress expect in return for funding ARPA-H at 2.5 billion dollars over three years.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
How will the agency figure out which projects to take on, especially with so many patient advocates for different diseases demanding moonshot funding for rapid progress?
I talked with Dr. Wegrzyn about the opportunities and challenges, what lessons ARPA-H is borrowing from Operation Warp Speed, how she decided on the first ARPA-H project that was announced recently, why a separate agency was needed instead of reforming HHS and the National Institutes of Health to be better at innovation, and how ARPA-H will make progress on disease prevention in addition to treatments for cancer, Alzheimer’s and diabetes, among many other health priorities.
Dr. Wegrzyn’s resume leaves no doubt of her suitability for this role. She was a program manager at DARPA where she focused on applying gene editing and synthetic biology to the goal of improving biosecurity. For her work there, she received the Superior Public Service Medal and, in case that wasn’t enough ARPA experience, she also worked at another ARPA that leads advanced projects in intelligence, called I-ARPA. Before that, she ran technical teams in the private sector working on gene therapies and disease diagnostics, among other areas. She has been a vice president of business development at Gingko Bioworks and headed innovation at Concentric by Gingko. Her training and education includes a PhD and undergraduate degree in applied biology from the Georgia Institute of Technology and she did her postdoc as an Alexander von Humboldt Fellow in Heidelberg, Germany.
Dr. Wegrzyn told me that she’s “in the hot seat.” The pressure is on for ARPA-H especially after the need and potential for health innovation was spot lit by the pandemic and the unprecedented speed of vaccine development. We'll soon find out if ARPA-H can produce gamechangers in health that are equivalent to DARPA’s creation of the internet.
Show links:
ARPA-H - https://arpa-h.gov/
Dr. Wegrzyn profile - https://arpa-h.gov/people/renee-wegrzyn/
Dr. Wegrzyn Twitter - https://twitter.com/rwegrzyn?lang=en
President Biden Announces Dr. Wegrzyn's appointment - https://www.whitehouse.gov/briefing-room/statement...
Leaps.org coverage of ARPA-H - https://leaps.org/arpa/
ARPA-H program for joints to heal themselves - https://arpa-h.gov/news/nitro/ -
ARPA-H virtual talent search - https://arpa-h.gov/news/aco-talent-search/

Dr. Renee Wegrzyn was appointed director of ARPA-H last October.
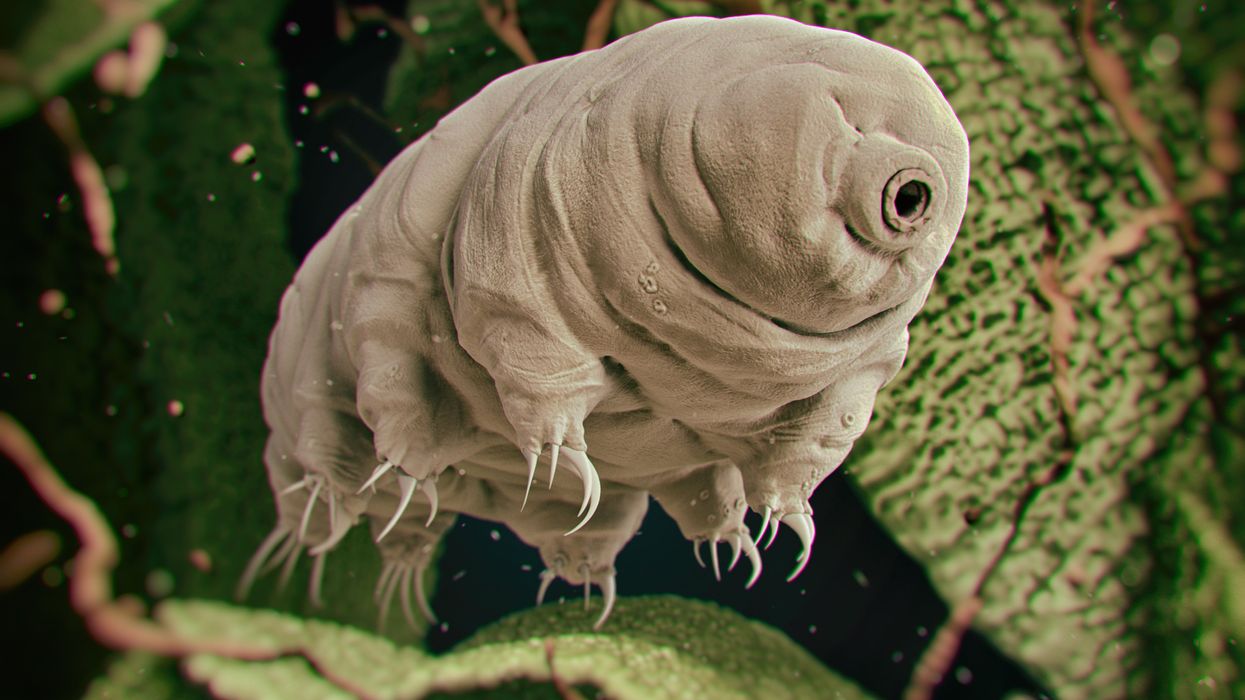
Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
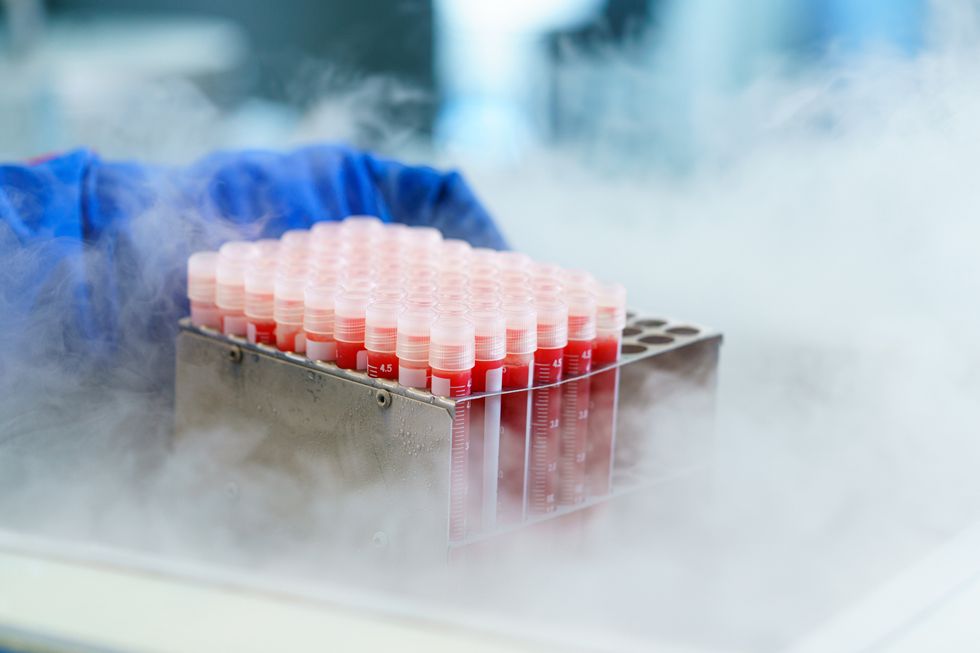
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.