More Families Are Using Nanny Cams to Watch Elderly Loved Ones, Raising Ethical Questions

Jackie Costanzo and her 93-year-old mom, Louise, are happy to have an extra way to stay connected with the camera, which is normally placed on a television stand facing her mom's bed.
After Jackie Costanzo's mother broke her right hip in a fall, she needed more hands-on care in her assisted-living apartment near Sacramento, California. A social worker from her health plan suggested installing a video camera to help ensure those services were provided.
Without the camera, Costanzo wouldn't have a way to confirm that caregivers had followed through with serving meals, changing clothes, and fulfilling other care needs.
When Costanzo placed the device in May 2018, she informed the administrator and staff, and at first, there were no objections. The facility posted a sign on the apartment's front door, alerting anyone who entered of recording in progress.
But this past spring, a new management company came across the sign and threatened to issue a 30-day eviction notice to her 93-year-old mother, Louise Munch, who has dementia, for violating a policy that prohibits cameras in residents' rooms. With encouragement from California Advocates for Nursing Home Reform, Costanzo researched the state's regulations but couldn't find anything to support or deny camera use. She refused to remove the recording device and prevailed.
"In essence, my mom was 'grandfathered in' because she moved in under a management company that did not specify that residents could not have cameras," says Costanzo, 73, a retired elementary schoolteacher who lives a three-hour drive away, in Silicon Valley, and visits one day every two weeks. Without the camera, Costanzo, who is her mother's only surviving child, wouldn't have a way to confirm that caregivers had followed through with serving meals, changing clothes, and fulfilling other care needs.
As technological innovations enable next of kin to remain apprised of the elderly's daily care in long-term care facilities, surveillance cameras bring legal and privacy issues to the forefront of a complex ethical debate. Families place them overtly or covertly—disguised in a makeshift clock radio, for instance—when they suspect or fear abuse or neglect, so they can maintain a watchful eye, perhaps deterring egregious behavior. But the cameras also capture intimate caregiving tasks, such as bathing and toileting, as well as dressing and undressing, which may undermine the dignity of residents.
So far, laws or guidelines in eight states—Illinois, Maryland, New Mexico, Oklahoma, Texas, Utah, Virginia, and Washington—have granted families the rights to install cameras in a resident's room. In addition, about 15 other states have proposed legislation. Some states, such as Pennsylvania, have put forth regulatory compliance guidance, according to a column published in the July/August 2018 issue of Annals of Long-Term Care.
The increasing prevalence of this legislation has placed it on the radar of long-term care providers. It also suggests a trend to clarify responsible camera use in monitoring services while respecting privacy, says Victor Lane Rose, the column's editor and director of aging services at ECRI Institute, a health care nonprofit near Philadelphia, Pennsylvania.
In most cases, a resident's family installs a camera or instigates a request in hopes of sparing their loved one from the harms of abuse, says James Wright, a family physician who serves as the ethics committee's vice chair of the Society for Post-Acute and Long-Term Care Medicine in Columbia, Maryland. A camera also allows the family to check in on the resident from afar and remain on alert for a potential fall or agitated state, he says.
"It's rare that a facility will have 24-hour presence in a patient's room. You won't have a nurse in there all the time," says Wright, who is also medical director of two long-term care centers and one assisted-living facility around Richmond, Virginia. Particularly "with dementia, the family often wonders" if their loved one is safe.
While offering families peace of mind, he notes that video cameras can also help exonerate caregivers accused of abuse or theft. Hearing aids, which typically cost between $2,000 and $3,000 each, often go missing. By reviewing a video together, families and administrators may find clues to a device's disappearance. Conversely, Wright empathizes with the main counterargument against camera use, which is the belief that "invasion of privacy is also invasion of human dignity."
In respecting modesty, ethical questions abound over whether a camera should be turned off when a patient is in the midst of receiving personal care, such as dressing and undressing or using bedpans. Other ethical issues revolve around who may access the recordings, says Lori Smetanka, executive director of the National Consumer Voice for Quality Long-Term Care in Washington, D.C.
Video cameras, she contends, are only one tool in shielding residents from abuse. They are "not substitutes for personal involvement," she says. "People need to be very vigilant visiting their family members, and facilities have a responsibility to ensure that residents are free of abuse."
Lack of accountability perpetuates abuse in long-term care settings and stems in large part from systemic underfunding.
Educating employees in abuse prevention becomes paramount, and families should ask about staff training before placing their loved one in a long-term care facility, Smetanka says. Prior to installing a camera, she recommends consulting an attorney who is familiar with this issue.
But thoughts of a camera often don't occur to families until an adverse event affects their loved one, says Toby Edelman, a senior policy attorney at the Center for Medicare Advocacy, a nonprofit organization with headquarters in Washington, D.C., and Connecticut.
"These cameras can show exactly what's going on," she explains, noting that prosecutors have used the recordings in litigation. "When residents have injuries of unknown origin" and they can't verbalize what happened to them, "the cameras may document that yes, the resident was actually hit by somebody."
With a resident's safety and security being "the most important consideration," the American Health Care Association in Washington, D.C., which represents long-term and post-acute care providers, supports allowing states, clinicians, and patients to decide about camera use on a local level, says David Gifford, senior vice president of quality and regulatory affairs and chief medical officer.
"We've seen some success with tools such as permissive legislation, where residents and their loved ones have the ability to determine whether a camera is right for them while working with the center openly and ensuring the confidentiality of other residents," says Gifford, who practiced as a geriatrician. "It is important to note, however, that surveillance cameras are still only one element of the quality matrix. We can never hope to truly improve quality care by catching bad actors after the fact."
Lack of accountability perpetuates abuse in long-term care settings and stems in large part from systemic underfunding. Low wages and morale are tied to high turnover, and cameras don't address this overarching problem, says Clara Berridge, an assistant professor of social work at the University of Washington in Seattle, who has co-authored articles on surveillance devices in elder care.
Employees often don't perceive a nursing assistant position as a long-term career trajectory and may not feel vested in the workplace. Training in the recognition and reporting of abuse becomes ineffective when workers quit shortly thereafter. Many must juggle multiple jobs to make ends meet. Staffing shortages are endemic, leading to inadequate oversight of residents and voicing of abuse complaints, she says.
In Berridge's assessment, cameras may do more harm than good. Respondents to a survey she conducted of nursing homes and assisted-living facilities in the United States found that recording devices tend to fuel workers' anxiety amid a culture that further demoralizes and dehumanizes the care they provide.
Consent becomes particularly thorny in shared rooms, which are more common than not in nursing homes. States that permit in-room cameras mandate that roommates or their legal representative be made aware. Even if the camera is directed away from their bed, it will still capture conversations as well as movements that enter its scope. "Surveillance isn't the best way to protect adults in need of support," Berridge says. "Public investment in quality care is."
"The camera is invaluable. But there's no law that says you can have it automatically, so that's wrong."
In the one-bedroom assisted-living apartment where Costanzo's mother lives alone, consent from another resident wasn't needed. Without a roommate, the camera is much less intrusive, although Costanzo wishes she had put one in the living room, not just the bedroom, for more security.
Her safety concerns escalated when she read about a Texas serial killer who smothered victims after gaining access to senior care facilities by "masquerading as a maintenance man." She points to such horrifying incidents, although exceedingly rare, as further justification for permitting cameras to help guard the vulnerable against abuse in long-term care settings. And she hopes to advocate for an applicable law in California.
"The camera is invaluable," says Costanzo, who pays for monthly Wi-Fi service so she can see and interact with her mother, who turns 94 in October, any time of day or night. "But there's no law that says you can have it automatically, so that's wrong."
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
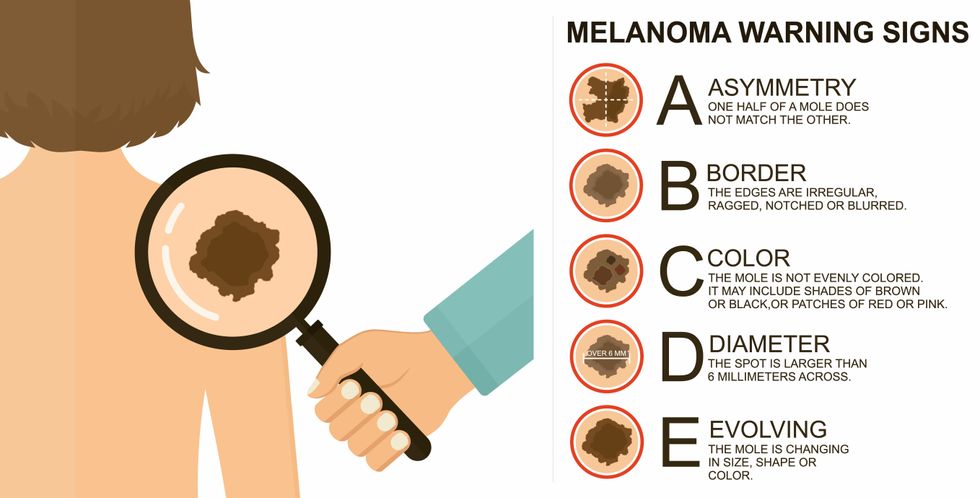
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

