New Options Are Emerging in the Search for Better Birth Control
A decade ago, Elizabeth Summers' options for birth control suddenly narrowed. Doctors diagnosed her with Factor V Leiden, a rare genetic disorder, after discovering blood clots in her lungs. The condition increases the risk of clotting, so physicians told Summers to stay away from the pill and other hormone-laden contraceptives. "Modern medicine has generally failed to provide me with an effective and convenient option," she says.
But new birth control options are emerging for women like Summers. These alternatives promise to provide more choices to women who can't ingest hormones or don't want to suffer their unpleasant side effects.
These new products have their own pros and cons. Still, doctors are welcoming new contraceptives following a long drought in innovation. "It's been a long time since we've had something new in the world of contraception," says Heather Irobunda, an obstetrician and gynecologist at NYC Health and Hospitals.
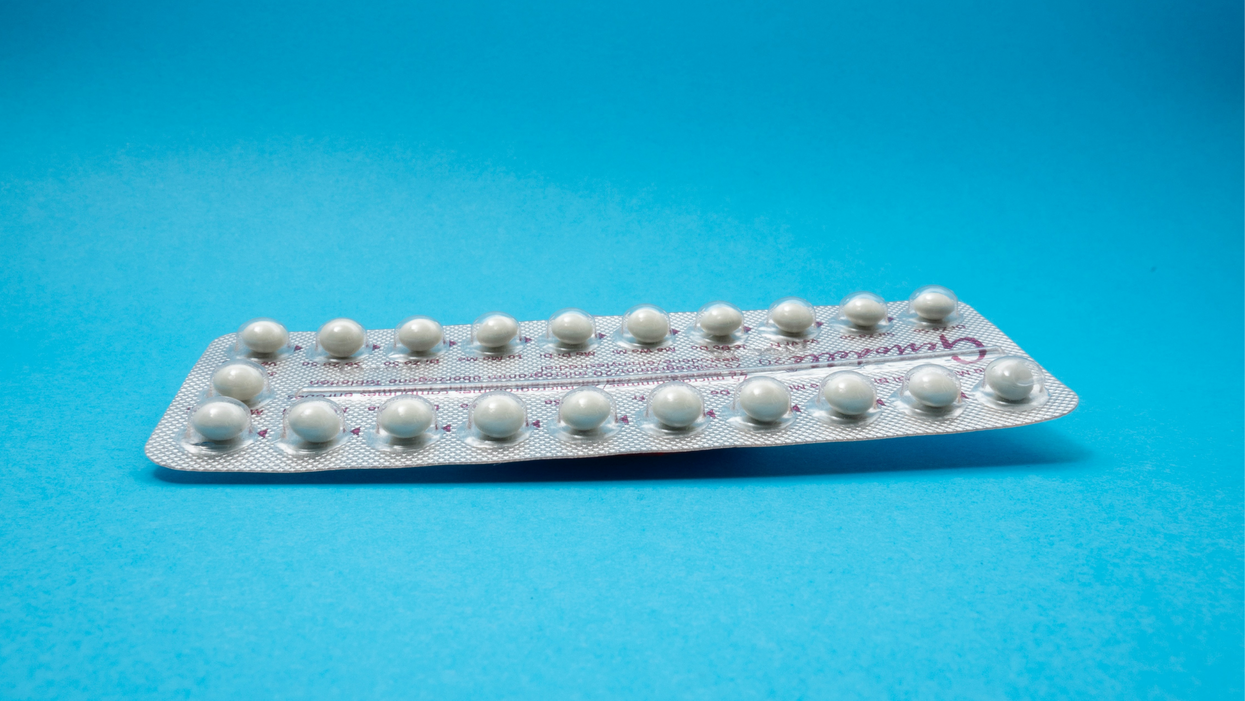
On social media, Irobunda often fields questions about one of these new options, a lubricating gel called Phexxi. San Diego-based Evofem, the company behind Phexxi, has been advertising the product on Hulu and Instagram after the gel was approved by the Food and Drug Administration in May 2020. The company's trendy ads target women who feel like condoms diminish the mood, but who also don't want to mess with an IUD or hormones.
Here's how it works: Phexxi is inserted via a tampon-like device up to an hour before sex. The gel regulates vaginal pH — essentially, the acidity levels — in a range that's inhospitable to sperm. It sounds a lot like spermicide, which is also placed in the vagina prior to sex to prevent pregnancy. But spermicide can damage the vagina's cell walls, which can increase the risk of contracting sexually transmitted diseases.
"Not only is innovation needed, but women want a non-hormonal option."
Phexxi isn't without side effects either. The most common one is vaginal burning, according to a late-stage trial. It's also possible to develop a urinary tract infection while using the product. That same study found that during typical use, Phexxi is about 86 percent effective at preventing pregnancy. The efficacy rate is comparable to condoms but lower than birth control pills (91 percent) and significantly lower than an IUD (99 percent).
Phexxi – which comes in a pack of 12 – represents a tiny but growing part of the birth control market. Pharmacies dispensed more than 14,800 packs from April through June this year, a 65 percent increase over the previous quarter, according to data from Evofem.
"We've been able to demonstrate that not only is innovation needed, but women want a non-hormonal option," says Saundra Pelletier, Evofem's CEO.
Beyond contraception, the company is carrying out late-stage tests to gauge Phexxi's effectiveness at preventing the sexually transmitted infections chlamydia and gonorrhea.

Phexxi is inserted via a tampon-like device up to an hour before sex.
Phexxi
A New Pill
The first birth control pill arrived in 1960, combining the hormones estrogen and progestin to stop sperm from joining with an egg, giving women control over their fertility. Subsequent formulations sought to ease side effects, by way of lower amounts of estrogen. But some women still experience headaches and nausea – or more serious complications like blood clots. On social media, women recently noted that birth control pills are much more likely to cause blood clots than Johnson & Johnson's COVID-19 vaccine that was briefly paused to evaluate the risk of clots in women under age 50. What will it take, they wondered, for safer birth control?
Mithra Pharmaceuticals of Belgium sought to create a gentler pill. In April, the FDA approved Mithra's Nextstellis, which includes a naturally occurring estrogen, the first new estrogen in the U.S. in 50 years. Nextstellis selectively acts on tissues lining the uterus, while other birth control pills have a broader target.
A Phase 3 trial showed a 98 percent efficacy rate. Andrew London, an obstetrician and gynecologist, who practices at several Maryland hospitals, says the results are in line with some other birth control pills. But, he added, early studies indicate that Nextstellis has a lower risk of blood clotting, along with other potential benefits, which additional clinical testing must confirm.
"It's not going to be worse than any other pill. We're hoping it's going to be significantly better," says London.
The estrogen in Nexstellis, called estetrol, was skipped over by the pharmaceutical industry after its discovery in the 1960s. Estetrol circulates between the mother and fetus during pregnancy. Decades later, researchers took a new look, after figuring out how to synthesize estetrol in a lab, as well as produce estetrol from plants.
"That allowed us to really start to investigate the properties and do all this stuff you have to do for any new drug," says Michele Gordon, vice president of marketing in women's health at Mayne Pharma, which licensed Nextstellis.
Bonnie Douglas, who followed the development of Nextstellis as part of a search for better birth control, recently switched to the product. "So far, it's much more tolerable," says Douglas. Previously, the Midwesterner was so desperate to find a contraceptive with fewer side effects that she turned to an online pharmacy to obtain a different birth control pill that had been approved in Canada but not in the U.S.
Contraceptive Access
Even if a contraceptive lands FDA approval, access poses a barrier. Getting insurers to cover new contraceptives can be difficult. For the uninsured, state and federal programs can help, and companies should keep prices in a reasonable range, while offering assistance programs. So says Kelly Blanchard, president of the nonprofit Ibis Reproductive Health. "For innovation to have impact, you want to reach as many folks as possible," she says.
In addition, companies developing new contraceptives have struggled to attract venture capital. That's changing, though.
In 2015, Sabrina Johnson founded DARÉ Bioscience around the idea of women's health. She estimated the company would be fully funded in six months, based on her track record in biotech and the demand for novel products.
But it's been difficult to get male investors interested in backing new contraceptives. It took Johnson two and a half years to raise the needed funds, via a reverse merger that took the company public. "There was so much education that was necessary," Johnson says, adding: "The landscape has changed considerably."
Johnson says she would like to think DARÉ had something to do with the shift, along with companies like Organon, a spinout of pharma company Merck that's focused on reproductive health. In surveying the fertility landscape, DARÉ saw limited non-hormonal options. On-demand options – like condoms – can detract from the moment. Copper IUDs must be inserted by a doctor and removed if a woman wants to return to fertility, and this method can have onerous side effects.
So, DARÉ created Ovaprene, a hormone-free device that's designed to be inserted into the vagina monthly by the user. The mesh product acts as a barrier, while releasing a chemical that immobilizes sperm. In an early study, the company reported that Ovaprene prevented almost all sperm from entering the cervical canal. The results, DARÉ believes, indicate high efficacy.
A late-stage study, slated to kick off next year, will be the true judge. Should Ovaprene eventually win regulatory approval, drug giant Bayer will handle commercializing the device.
Other new forms of birth control in development are further out, and that's assuming they perform well in clinical trials. Among them: a once-a-month birth control pill, along with a male version of the birth control pill. The latter is often brought up among women who say it's high time that men take a more proactive role in birth control.
For Summers, her search for a safe and convenient birth control continues. She tried Phexxi, which caused irritation. Still, she's excited that a non-hormonal option now exists. "I'm sure it will work for others," she says.
Study of the DNA double helix will lead to transformative medical care and increasingly urgent questions about how to responsibly handle genetic engineering technology.
The news last November that a rogue Chinese scientist had genetically altered the embryos of a pair of Chinese twins shocked the world. But although this use of advanced technology to change the human gene pool was premature, it was a harbinger of how genetic science will alter our healthcare, the way we make babies, the nature of the babies we make, and, ultimately, our sense of who and what we are as a species.
The healthcare applications of the genetics revolution are merely stations along the way to the ultimate destination.
But while the genetics revolution has already begun, we aren't prepared to handle these Promethean technologies responsibly.
By identifying the structure of DNA in the 1950s, Watson, Crick, Wilkins, and Franklin showed that the book of life was written in the DNA double helix. When the human genome project was completed in 2003, we saw how this book of human life could be transcribed. Painstaking research paired with advanced computational algorithms then showed what increasing numbers of genes do and how the genetic book of life can be read.
Now, with the advent of precision gene editing tools like CRISPR, we are seeing that the book of life -- and all biology -- can be re-written. Biology is being recognized as another form of readable, writable, and hackable information technology with we humans as the coders.
The impact of this transformation is being first experienced in our healthcare. Gene therapies including those extracting, re-engineering, then reintroducing a person's own cells enhanced into cancer-fighting supercells are already performing miracles in clinical trials. Thousands of applications have already been submitted to regulators across the globe for trials using gene therapies to address a host of other diseases.
Recently, the first gene editing of cells inside a person's body was deployed to treat the genetically relatively simple metabolic disorder Hunter syndrome, with many more applications to come. These new approaches are only the very first steps in our shift from the current system of generalized medicine based on population averages to precision medicine based on each patient's individual biology to predictive medicine based on AI-generated estimations of a person's future health state.

Jamie Metzl's groundbreaking new book, Hacking Darwin: Genetic Engineering and the Future of Humanity, explores how the genetic revolution is transforming our healthcare, the way we make babies, and the nature of and babies we make, what this means for each of us, and what we must all do now to prepare for what's coming.
This shift in our healthcare will ensure that millions and then billions of people will have their genomes sequenced as the foundation of their treatment. Big data analytics will then be used to compare at scale people's genotypes (what their genes say) to their phenotypes (how those genes are expressed over the course of their lives).
These massive datasets of genetic and life information will then make it possible to go far beyond the simple genetic analysis of today and to understand far more complex human diseases and traits influenced by hundreds or thousands of genes. Our understanding of this complex genetic system within the vaster ecosystem of our bodies and the environment around us will transform healthcare for the better and help us cure terrible diseases that have plagued our ancestors for millennia.
But as revolutionary as this challenge will be for medicine, the healthcare applications of the genetics revolution are merely stations along the way to the ultimate destination – a deep and fundamental transformation of our evolutionary trajectory as a species.
A first inkling of where we are heading can be seen in the direct-to-consumer genetic testing industry. Many people around the world have now sent their cheek swabs to companies like 23andMe for analysis. The information that comes back can tell people a lot about relatively simple genetic traits like carrier status for single gene mutation diseases, eye color, or whether they hate the taste of cilantro, but the information about complex traits like athletic predisposition, intelligence, or personality style today being shared by some of these companies is wildly misleading.
This will not always be the case. As the genetic and health data pools grow, analysis of large numbers of sequenced genomes will make it possible to apply big data analytics to predict some very complex genetic disease risks and the genetic components of traits like height, IQ, temperament, and personality style with increasing accuracy. This process, called "polygenic scoring," is already being offered in beta stage by a few companies and will become an ever bigger part of our lives going forward.
The most profound application of all this will be in our baby-making. Before making a decision about which of the fertilized eggs to implant, women undergoing in vitro fertilization can today elect to have a small number of cells extracted from their pre-implanted embryos and sequenced. With current technology, this can be used to screen for single-gene mutation diseases and other relatively simple disorders. Polygenic scoring, however, will soon make it possible to screen these early stage pre-implanted embryos to assess their risk of complex genetic diseases and even to make predictions about the heritable parts of complex human traits. The most intimate elements of being human will start feeling like high-pressure choices needing to be made by parents.
The limit of our imagination will become the most significant barrier to our recasting biology.
Adult stem cell technologies will then likely make it possible to generate hundreds or thousands of a woman's own eggs from her blood sample or skin graft. This would blow open the doors of reproductive possibility and allow parents to choose embryos with exceptional potential capabilities from a much larger set of options.
The complexity of human biology will place some limits to the extent of possible gene edits that might be made to these embryos, but all of biology, including our own, is extremely flexible. How else could all the diversity of life have emerged from a single cell nearly four billion years ago? The limit of our imagination will become the most significant barrier to our recasting biology.
But while we humans are gaining the powers of the gods, we aren't at all ready to use them.
The same tools that will help cure our worst afflictions, save our children, help us live longer, healthier, more robust lives will also open the door to potential abuses. Prospective parents with the best of intentions or governments with lax regulatory structures or aggressive ideas of how population-wide genetic engineering might be used to enhance national competitiveness or achieve some other goal could propel us into a genetic arms race that could undermine our essential diversity, dangerously divide societies, lead to dangerous, destabilizing, and potentially even deadly conflicts between us, and threaten our very humanity.
But while the advance of genetic technologies is inevitable, how it plays out is anything but. If we don't want the genetic revolution to undermine our species or lead to grave conflicts between genetic haves and have nots or between societies opting in and those opting out, now is the time when we need to make smart decisions based on our individual and collective best values. Although the technology driving the genetic revolution is new, the value systems we will need to optimize the benefits and minimize the harms of this massive transformation are ones we have been developing for thousands of years.
And while some very smart and well-intentioned scientists have been meeting to explore what comes next, it won't be enough for a few of even our wisest prophets to make decisions about the future of our species that will impact everyone. We'll also need smart regulations on both the national and international levels.
Every country will need to have its own regulatory guidelines for human genetic engineering based on both international best practices and the country's unique traditions and values. Because we are all one species, however, we will also ultimately need to develop guidelines that can apply to all of us.
As a first step toward making this possible, we must urgently launch a global, species-wide education effort and inclusive dialogue on the future of human genetic engineering that can eventually inform global norms that will need to underpin international regulations. This process will not be easy, but the alternative of an unregulated genetic arms race would be far worse.
The overlapping genomics and AI revolutions may seem like distant science fiction but are closer than you think. Far sooner than most people recognize, the inherent benefits of these technologies and competition between us will spark rapid adoption. Before that spark ignites, we have a brief moment to come together as a species like we never have before to articulate and translate into action the future we jointly envision. The north star of our best shared values can help us navigate the almost unimaginable opportunities and very real challenges that lie ahead.
Edible water bubble pods, called Ooho, made by Skipping Rock Labs. (Photo credit: Instagram account of Ooho water)
Here's something to chew on. Can a gulp of water help save the planet? If you're drinking *and* eating your water at the same time, the answer may be yes.
The tasteless packaging is made from brown seaweed that biodegrades naturally in four to six weeks.
The Lowdown
A start-up company called Skipping Rocks Lab has created a "water bubble" encased in an edible sachet that you can pop in your mouth whole. Or if you're not into swallowing it, you can tear off the edge, drink up, and toss the rest in a composter. The tasteless packaging is made from brown seaweed that biodegrades naturally in four to six weeks, whereas plastic water bottles can linger for hundreds of years.
The founders of the London-based company are determined to "make plastic packaging disappear." They had two foodie inspirations: molecular gastronomists and fruit. They tried to emulate the way chefs used edible membranes to encase bubbles of liquid to make things like fake caviar and fake egg yolks; and they also considered the peel of an orange or banana, which protects the tasty insides but can be composted.
The sachets can also contain other liquids that come in single-serve plastic containers -- think packets of condiments with takeout meals, specialty cocktails at parties, and especially single servings of water for sporting events. The London Marathon last month gave out the water bubble pods at a station along the route, using them to replace 200,000 plastic bottles that would have likely ended up first in the street, and ultimately in the ocean.
Next Up
The engineers and chemists at Skipping Rocks intend to lease their machines to others who can then manufacture their own sachets on-site to fill with whatever they desire. The new material, which is dubbed "Notpla" (not plastic), also has other applications beyond holding liquids. It can be used to replace the plastic lining in cardboard takeout boxes, for example. And the startup is working on additional materials to replace other types of ubiquitous plastic packaging, like the netting that encases garlic and onions, and the sachets that hold nails and screws.
Edible water bubbles may be the future of drinks at sporting events and festivals.
Open Questions
One hurdle is that the pods are not very hardy, so while they work fine to hand out along a marathon route, they wouldn't really be viable for a hiker to throw in her backpack. Another issue concerns the retail market: to be stable on a shelf, they'd have to be protected from all that handling, which brings us back to the problem the engineers tried to solve in the first place -- disposable packaging.
So while Skipping Rocks may not achieve their ultimate goal of ridding the world of plastic waste, a little progress can still go a long way. If edible water bubbles are the future of drinks at sporting events and festivals, the environment will certainly benefit from their presence -- and absence.

