One Day, There Might Be a Drug for a Broken Heart

A sad woman peering through sunlit blinds.
For Tony Y., 37, healing from heartbreak is slow and incomplete. Each of several exes is associated with a cluster of sore memories. Although he loves the Blue Ridge Mountains, he can't visit because they remind him of a romantic holiday years ago.
If a new drug made rejections less painful, one expert argues, it could relieve or even prevent major depression.
Like some 30 to 40 percent of depressed patients, Tony hasn't had success with current anti-depressants. One day, psychiatrists may be able to offer him a new kind of opioid, an anti-depressant for people suffering from the cruel pain of rejection.
A Surprising Discovery
As we move through life, rejections -- bullying in school, romantic breakups, and divorces -- are powerful triggers to depressive episodes, observes David Hsu, a neuroscientist at Stony Brook University School of Medicine in Long Island, New York. If a new drug made them less painful, he argues, it could relieve or even prevent major depression.
Our bodies naturally produce opioids to soothe physical pain, and opioid drugs like morphine and oxycodone work by plugging into the same receptors in our brains. The same natural opioids may also respond to emotional hurts, and painkillers can dramatically affect mood. Today's epidemic of opioid abuse raises the question: How many lives might have been saved if we had a safe, non-addictive option for medicating emotional pain?
Already one anti-depressant, tianeptine, locks into the mu opioid receptor, the target of morphine and oxycodone. Scientists knew that tianeptine, prescribed in some countries in Europe, Asia, and Latin America, acted differently than the most common anti-depressants in use today, which affect the levels of other brain chemicals, serotonin and norepinephrine. But the discovery in 2014 that tianeptine tapped the mu receptor was a "huge surprise," says co-author Jonathan Javitch, chief of the Division of Molecular Therapeutics at Columbia University.
The news arrived when scientists' basic understanding of depression is in flux; viewed biologically, it may cover several disorders. One of them could hinge on opioids. It's possible that some people release fewer opioids naturally or that the receptors for it are less effective.
Javitch has launched a startup, Kures, to make tianeptine more effective and convenient and to find other opioid-modulators. That may seem quixotic in the midst of an opioid epidemic, but tianeptine doesn't create dependency in low, prescription doses and has been used safely around the world for decades. To identify likely patients, cofounder Andrew Kruegel is looking for ways to "segment the depressed population by measures that have to do with opioid release," he says.
Is Emotional Pain Actually "Pain"?
No one imagines that the pain from rejection or loss is the same as pain from a broken leg. Physical pain is two perceptions—a sensory perception and an "affective" one, which makes pain unpleasant.
Exploration of an overlap between physical and what research psychologists call "social pain" has heated up since the mid-2000s.
The sensory perception, processed by regions of the brain called the primary and secondary somatosensory cortices and the posterior insula, tells us whether the pain is in your arm or your leg, how strong it is and whether it is a sting, ache, or has some other quality. The affective perception, in another part of the brain called the dorsal anterior cingulate cortex and the anterior insula, tells us that we want the pain to stop, fast! When people with lesions in the latter areas experience a stimulus that ordinarily would be painful, they don't mind it.
Science now suggests that emotional pain arises in the affective brain circuits. Exploration of an overlap between physical and what research psychologists call "social pain" has heated up since the mid-2000s. Animal evidence goes back to the 1970s: babies separated from their mothers showed less distress when given morphine, and more if dosed with naloxone, the opioid antagonist.
Parents, of course, face the question of whether Baby feels alone or wet whenever she howls. And the answer is: both hurt. Being abandoned is the ultimate threat in our early life, and it makes sense that a brain system to monitor social threats would piggyback upon an existing system for pain. Piggybacking is a feature of evolution. An ancestor who felt "hurt" when threatened by rejection might learn adaptive behavior: to cooperate or run.
In 2010, a large multi-university team led by Nathan DeWall at the University of Kentucky, reported that acetaminophen (Tylenol) reduced social pain. Undergraduates took 500 mg of acetaminophen upon awakening and at bedtime every day for three weeks and reported nightly about their day using a previously-tested "Hurt Feelings Scale," rating how strongly they agreed with questions like, "Today, being teased hurt my feelings."
Over the weeks, their reports of hurt feelings steadily declined, while remaining flat in a control group that took placebos. In a second experiment, the research group showed that, compared to controls, people who had taken acetaminophen for three weeks showed less brain activity in the affective brain circuits while they experienced rejection during a virtual ball-tossing game. Later, Hsu's brain scan research supported the idea that rejection triggers the mu opioid receptor system, which normally provides pain-dampening opioids.
More evidence comes from nonhuman primates with lesions in the affective circuits: They cry less when separated from caregivers or social groups.
Heartbreak seems to lie in those regions: women with major depression are more hurt by romantic rejection than normal controls are and show more activity in those areas in brain scans, Hsu found. Also, factors that make us more vulnerable to rejection -- like low self-esteem -- are linked to more activity in the key areas, studies show.
The trait "high rejection sensitivity" increases your risk of depression more than "global neuroticism" does, Hsu observes, and predicts a poor recovery from depression. Pain sensitivity is another clue: People with a gene linked to it seem to be more hurt by social exclusion. Once you're depressed, you become more rejection-sensitive and prone to pain—a classic bad feedback loop.
"Ideally, we'd have biomarkers to distinguish when loss becomes complicated grief and then depression, and we might prevent the transition with a drug."
Helen Mayberg, a neurologist renowned for her study of brain circuits in depression, sees, as Hsu does, the possibility of preventing depressions. "Nobody would suggest we treat routine bad social pain with drugs. But it is true that in susceptible people, losing a partner, for example, can lead to a full-blown depression," says Mayberg, who is the founding director of The Center for Advanced Circuit Therapeutics at Mount Sinai's Icahn School of Medicine in New York City. "Ideally, we'd have biomarkers to distinguish when loss becomes complicated grief and then depression, and we might prevent the transition with a drug. It would be like taking medication when you feel the warning symptoms of a headache to prevent a full-blown migraine."
A Way Out of the Opioid Crisis?
The exploration of social pain should lead us to a deeper understanding of pain, beyond the sharp distinctions between "physical" and "psychological." Finding our way out of the current crisis may require that deeper understanding. About half of the people with opioid prescriptions have mental health disorders. "I expect there are a lot of people using street opioids—heroin or prescriptions purchased from others--to self-medicate psychological pain," Kreugel says.
What we may need, he suggests, is "a new paradigm for using opioids in psychiatry: low, sub-analgesic, sub-euphoric dosing." But so far it hasn't been easy. Investors don't flock to fund psychiatric drugs and in 2018, the word opioid is poison.
As for Tony Y., he's struggled for three years to recover from his most serious relationship. "Driving around highways looking at exit signs toward places we visited together sometimes fills me with unbearable anguish," he admits. "And because we used to do so much bird watching together, sometimes a mere glimpse of a random bird sets me off." He perks up at the idea of a heartbreak drug. "If the side effects didn't seem bad, I would consider it, absolutely."
A movie still from the 1966 film "Fantastic Voyage"
In the 1966 movie "Fantastic Voyage," actress Raquel Welch and her submarine were shrunk to the size of a cell in order to eliminate a blood clot in a scientist's brain. Now, 55 years later, the scenario is becoming closer to reality.
California-based startup Bionaut Labs has developed a nanobot about the size of a grain of rice that's designed to transport medication to the exact location in the body where it's needed. If you think about it, the conventional way to deliver medicine makes little sense: A painkiller affects the entire body instead of just the arm that's hurting, and chemotherapy is flushed through all the veins instead of precisely targeting the tumor.
"Chemotherapy is delivered systemically," Bionaut-founder and CEO Michael Shpigelmacher says. "Often only a small percentage arrives at the location where it is actually needed."
But what if it was possible to send a tiny robot through the body to attack a tumor or deliver a drug at exactly the right location?
Several startups and academic institutes worldwide are working to develop such a solution but Bionaut Labs seems the furthest along in advancing its invention. "You can think of the Bionaut as a tiny screw that moves through the veins as if steered by an invisible screwdriver until it arrives at the tumor," Shpigelmacher explains. Via Zoom, he shares the screen of an X-ray machine in his Culver City lab to demonstrate how the half-transparent, yellowish device winds its way along the spine in the body. The nanobot contains a tiny but powerful magnet. The "invisible screwdriver" is an external magnetic field that rotates that magnet inside the device and gets it to move and change directions.
The current model has a diameter of less than a millimeter. Shpigelmacher's engineers could build the miniature vehicle even smaller but the current size has the advantage of being big enough to see with bare eyes. It can also deliver more medicine than a tinier version. In the Zoom demonstration, the micorobot is injected into the spine, not unlike an epidural, and pulled along the spine through an outside magnet until the Bionaut reaches the brainstem. Depending which organ it needs to reach, it could be inserted elsewhere, for instance through a catheter.
"The hope is that we can develop a vehicle to transport medication deep into the body," says Max Planck scientist Tian Qiu.
Imagine moving a screw through a steak with a magnet — that's essentially how the device works. But of course, the Bionaut is considerably different from an ordinary screw: "At the right location, we give a magnetic signal, and it unloads its medicine package," Shpigelmacher says.
To start, Bionaut Labs wants to use its device to treat Parkinson's disease and brain stem gliomas, a type of cancer that largely affects children and teenagers. About 300 to 400 young people a year are diagnosed with this type of tumor. Radiation and brain surgery risk damaging sensitive brain tissue, and chemotherapy often doesn't work. Most children with these tumors live less than 18 months. A nanobot delivering targeted chemotherapy could be a gamechanger. "These patients really don't have any other hope," Shpigelmacher says.
Of course, the main challenge of the developing such a device is guaranteeing that it's safe. Because tissue is so sensitive, any mistake could risk disastrous results. In recent years, Bionaut has tested its technology in dozens of healthy sheep and pigs with no major adverse effects. Sheep make a good stand-in for humans because their brains and spines are similar to ours.

The Bionaut device is about the size of a grain of rice.
Bionaut Labs
"As the Bionaut moves through brain tissue, it creates a transient track that heals within a few weeks," Shpigelmacher says. The company is hoping to be the first to test a nanobot in humans. In December 2022, it announced that a recent round of funding drew $43.2 million, for a total of 63.2 million, enabling more research and, if all goes smoothly, human clinical trials by early next year.
Once the technique has been perfected, further applications could include addressing other kinds of brain disorders that are considered incurable now, such as Alzheimer's or Huntington's disease. "Microrobots could serve as a bridgehead, opening the gateway to the brain and facilitating precise access of deep brain structure – either to deliver medication, take cell samples or stimulate specific brain regions," Shpigelmacher says.
Robot-assisted hybrid surgery with artificial intelligence is already used in state-of-the-art surgery centers, and many medical experts believe that nanorobotics will be the instrument of the future. In 2016, three scientists were awarded the Nobel Prize in Chemistry for their development of "the world's smallest machines," nano "elevators" and minuscule motors. Since then, the scientific experiments have progressed to the point where applicable devices are moving closer to actually being implemented.
Bionaut's technology was initially developed by a research team lead by Peer Fischer, head of the independent Micro Nano and Molecular Systems Lab at the Max Planck Institute for Intelligent Systems in Stuttgart, Germany. Fischer is considered a pioneer in the research of nano systems, which he began at Harvard University more than a decade ago. He and his team are advising Bionaut Labs and have licensed their technology to the company.
"The hope is that we can develop a vehicle to transport medication deep into the body," says Max Planck scientist Tian Qiu, who leads the cooperation with Bionaut Labs. He agrees with Shpigelmacher that the Bionaut's size is perfect for transporting medication loads and is researching potential applications for even smaller nanorobots, especially in the eye, where the tissue is extremely sensitive. "Nanorobots can sneak through very fine tissue without causing damage."
In "Fantastic Voyage," Raquel Welch's adventures inside the body of a dissident scientist let her swim through his veins into his brain, but her shrunken miniature submarine is attacked by antibodies; she has to flee through the nerves into the scientist's eye where she escapes into freedom on a tear drop. In reality, the exit in the lab is much more mundane. The Bionaut simply leaves the body through the same port where it entered. But apart from the dramatization, the "Fantastic Voyage" was almost prophetic, or, as Shpigelmacher says, "Science fiction becomes science reality."
This article was first published by Leaps.org on April 12, 2021.
How the Human Brain Project Built a Mind of its Own
In 2013, the Human Brain Project set out to build a realistic computer model of the brain over ten years. Now, experts are reflecting on HBP's achievements with an eye toward the future.
In 2009, neuroscientist Henry Markram gave an ambitious TED talk. “Our mission is to build a detailed, realistic computer model of the human brain,” he said, naming three reasons for this unmatched feat of engineering. One was because understanding the human brain was essential to get along in society. Another was because experimenting on animal brains could only get scientists so far in understanding the human ones. Third, medicines for mental disorders weren’t good enough. “There are two billion people on the planet that are affected by mental disorders, and the drugs that are used today are largely empirical,” Markram said. “I think that we can come up with very concrete solutions on how to treat disorders.”
Markram's arguments were very persuasive. In 2013, the European Commission launched the Human Brain Project, or HBP, as part of its Future and Emerging Technologies program. Viewed as Europe’s chance to try to win the “brain race” between the U.S., China, Japan, and other countries, the project received about a billion euros in funding with the goal to simulate the entire human brain on a supercomputer, or in silico, by 2023.
Now, after 10 years of dedicated neuroscience research, the HBP is coming to an end. As its many critics warned, it did not manage to build an entire human brain in silico. Instead, it achieved a multifaceted array of different goals, some of them unexpected.
Scholars have found that the project did help advance neuroscience more than some detractors initially expected, specifically in the area of brain simulations and virtual models. Using an interdisciplinary approach of combining technology, such as AI and digital simulations, with neuroscience, the HBP worked to gain a deeper understanding of the human brain’s complicated structure and functions, which in some cases led to novel treatments for brain disorders. Lastly, through online platforms, the HBP spearheaded a previously unmatched level of global neuroscience collaborations.
Simulating a human brain stirs up controversy
Right from the start, the project was plagued with controversy and condemnation. One of its prominent critics was Yves Fregnac, a professor in cognitive science at the Polytechnic Institute of Paris and research director at the French National Centre for Scientific Research. Fregnac argued in numerous articles that the HBP was overfunded based on proposals with unrealistic goals. “This new way of over-selling scientific targets, deeply aligned with what modern society expects from mega-sciences in the broad sense (big investment, big return), has been observed on several occasions in different scientific sub-fields,” he wrote in one of his articles, “before invading the field of brain sciences and neuromarketing.”
"A human brain model can simulate an experiment a million times for many different conditions, but the actual human experiment can be performed only once or a few times," said Viktor Jirsa, a professor at Aix-Marseille University.
Responding to such critiques, the HBP worked to restructure the effort in its early days with new leadership, organization, and goals that were more flexible and attainable. “The HBP got a more versatile, pluralistic approach,” said Viktor Jirsa, a professor at Aix-Marseille University and one of the HBP lead scientists. He believes that these changes fixed at least some of HBP’s issues. “The project has been on a very productive and scientifically fruitful course since then.”
After restructuring, the HBP became a European hub on brain research, with hundreds of scientists joining its growing network. The HBP created projects focused on various brain topics, from consciousness to neurodegenerative diseases. HBP scientists worked on complex subjects, such as mapping out the brain, combining neuroscience and robotics, and experimenting with neuromorphic computing, a computational technique inspired by the human brain structure and function—to name just a few.
Simulations advance knowledge and treatment options
In 2013, it seemed that bringing neuroscience into a digital age would be farfetched, but research within the HBP has made this achievable. The virtual maps and simulations various HBP teams create through brain imaging data make it easier for neuroscientists to understand brain developments and functions. The teams publish these models on the HBP’s EBRAINS online platform—one of the first to offer access to such data to neuroscientists worldwide via an open-source online site. “This digital infrastructure is backed by high-performance computers, with large datasets and various computational tools,” said Lucy Xiaolu Wang, an assistant professor in the Resource Economics Department at the University of Massachusetts Amherst, who studies the economics of the HBP. That means it can be used in place of many different types of human experimentation.
Jirsa’s team is one of many within the project that works on virtual brain models and brain simulations. Compiling patient data, Jirsa and his team can create digital simulations of different brain activities—and repeat these experiments many times, which isn’t often possible in surgeries on real brains. “A human brain model can simulate an experiment a million times for many different conditions,” Jirsa explained, “but the actual human experiment can be performed only once or a few times.” Using simulations also saves scientists and doctors time and money when looking at ways to diagnose and treat patients with brain disorders.

Compiling patient data, scientists can create digital simulations of different brain activities—and repeat these experiments many times.
The Human Brain Project
Simulations can help scientists get a full picture that otherwise is unattainable. “Another benefit is data completion,” added Jirsa, “in which incomplete data can be complemented by the model. In clinical settings, we can often measure only certain brain areas, but when linked to the brain model, we can enlarge the range of accessible brain regions and make better diagnostic predictions.”
With time, Jirsa’s team was able to move into patient-specific simulations. “We advanced from generic brain models to the ability to use a specific patient’s brain data, from measurements like MRI and others, to create individualized predictive models and simulations,” Jirsa explained. He and his team are working on this personalization technique to treat patients with epilepsy. According to the World Health Organization, about 50 million people worldwide suffer from epilepsy, a disorder that causes recurring seizures. While some epilepsy causes are known others remain an enigma, and many are hard to treat. For some patients whose epilepsy doesn’t respond to medications, removing part of the brain where seizures occur may be the only option. Understanding where in the patients’ brains seizures arise can give scientists a better idea of how to treat them and whether to use surgery versus medications.
“We apply such personalized models…to precisely identify where in a patient’s brain seizures emerge,” Jirsa explained. “This guides individual surgery decisions for patients for which surgery is the only treatment option.” He credits the HBP for the opportunity to develop this novel approach. “The personalization of our epilepsy models was only made possible by the Human Brain Project, in which all the necessary tools have been developed. Without the HBP, the technology would not be in clinical trials today.”
Personalized simulations can significantly advance treatments, predict the outcome of specific medical procedures and optimize them before actually treating patients. Jirsa is watching this happen firsthand in his ongoing research. “Our technology for creating personalized brain models is now used in a large clinical trial for epilepsy, funded by the French state, where we collaborate with clinicians in hospitals,” he explained. “We have also founded a spinoff company called VB Tech (Virtual Brain Technologies) to commercialize our personalized brain model technology and make it available to all patients.”
The Human Brain Project created a level of interconnectedness within the neuroscience research community that never existed before—a network not unlike the brain’s own.
Other experts believe it’s too soon to tell whether brain simulations could change epilepsy treatments. “The life cycle of developing treatments applicable to patients often runs over a decade,” Wang stated. “It is still too early to draw a clear link between HBP’s various project areas with patient care.” However, she admits that some studies built on the HBP-collected knowledge are already showing promise. “Researchers have used neuroscientific atlases and computational tools to develop activity-specific stimulation programs that enabled paraplegic patients to move again in a small-size clinical trial,” Wang said. Another intriguing study looked at simulations of Alzheimer’s in the brain to understand how it evolves over time.
Some challenges remain hard to overcome even with computer simulations. “The major challenge has always been the parameter explosion, which means that many different model parameters can lead to the same result,” Jirsa explained. An example of this parameter explosion could be two different types of neurodegenerative conditions, such as Parkinson’s and Huntington’s diseases. Both afflict the same area of the brain, the basal ganglia, which can affect movement, but are caused by two different underlying mechanisms. “We face the same situation in the living brain, in which a large range of diverse mechanisms can produce the same behavior,” Jirsa said. The simulations still have to overcome the same challenge.
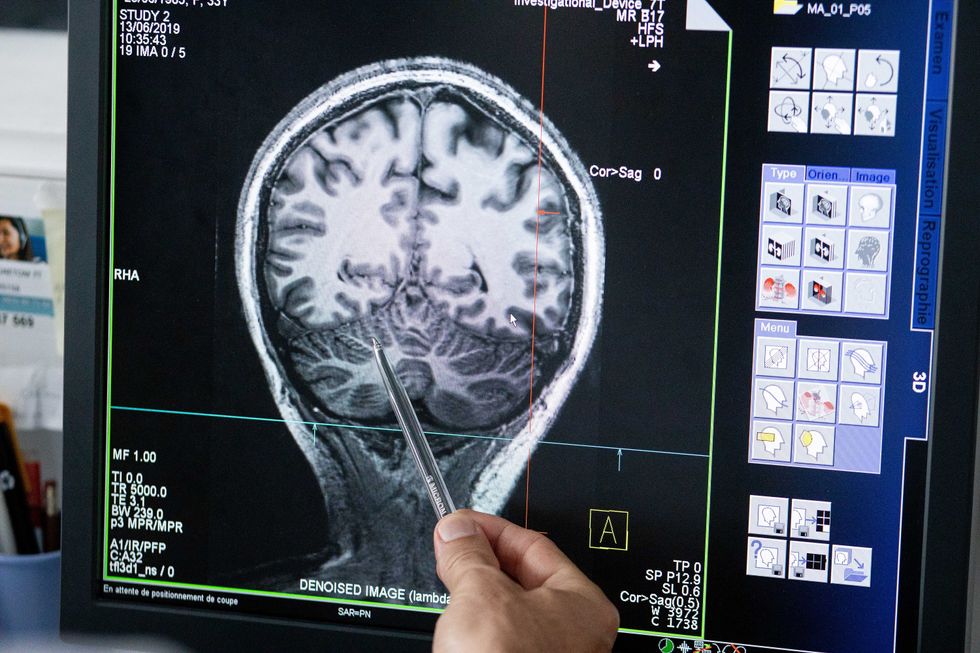
Understanding where in the patients’ brains seizures arise can give scientists a better idea of how to treat them and whether to use surgery versus medications.
The Human Brain Project
A network not unlike the brain’s own
Though the HBP will be closing this year, its legacy continues in various studies, spin-off companies, and its online platform, EBRAINS. “The HBP is one of the earliest brain initiatives in the world, and the 10-year long-term goal has united many researchers to collaborate on brain sciences with advanced computational tools,” Wang said. “Beyond the many research articles and projects collaborated on during the HBP, the online neuroscience research infrastructure EBRAINS will be left as a legacy even after the project ends.”
Those who worked within the HBP see the end of this project as the next step in neuroscience research. “Neuroscience has come closer to very meaningful applications through the systematic link with new digital technologies and collaborative work,” Jirsa stated. “In that way, the project really had a pioneering role.” It also created a level of interconnectedness within the neuroscience research community that never existed before—a network not unlike the brain’s own. “Interconnectedness is an important advance and prerequisite for progress,” Jirsa said. “The neuroscience community has in the past been rather fragmented and this has dramatically changed in recent years thanks to the Human Brain Project.”
According to its website, by 2023 HBP’s network counted over 500 scientists from over 123 institutions and 16 different countries, creating one of the largest multi-national research groups in the world. Even though the project hasn’t produced the in-silico brain as Markram envisioned it, the HBP created a communal mind with immense potential. “It has challenged us to think beyond the boundaries of our own laboratories,” Jirsa said, “and enabled us to go much further together than we could have ever conceived going by ourselves.”