Some hospitals are pioneers in ditching plastic, turning green

In the U.S., hospitals generate an estimated 6,000 tons of waste per day. A few clinics are leading the way in transitioning to clean energy sources.
This is part 2 of a three part series on a new generation of doctors leading the charge to make the health care industry more sustainable - for the benefit of their patients and the planet. Read part 1 here and part 3 here.
After graduating from her studies as an engineer, Nora Stroetzel ticked off the top item on her bucket list and traveled the world for a year. She loved remote places like the Indonesian rain forest she reached only by hiking for several days on foot, mountain villages in the Himalayas, and diving at reefs that were only accessible by local fishing boats.
“But no matter how far from civilization I ventured, one thing was already there: plastic,” Stroetzel says. “Plastic that would stay there for centuries, on 12,000 foot peaks and on beaches several hundred miles from the nearest city.” She saw “wild orangutans that could be lured by rustling plastic and hermit crabs that used plastic lids as dwellings instead of shells.”
While traveling she started volunteering for beach cleanups and helped build a recycling station in Indonesia. But the pivotal moment for her came after she returned to her hometown Kiel in Germany. “At the dentist, they gave me a plastic cup to rinse my mouth. I used it for maybe ten seconds before it was tossed out,” Stroetzel says. “That made me really angry.”
She decided to research alternatives for plastic in the medical sector and learned that cups could be reused and easily disinfected. All dentists routinely disinfect their tools anyway and, Stroetzel reasoned, it wouldn’t be too hard to extend that practice to cups.
It's a good example for how often plastic is used unnecessarily in medical practice, she says. The health care sector is the fifth biggest source of pollution and trash in industrialized countries. In the U.S., hospitals generate an estimated 6,000 tons of waste per day, including an average of 400 grams of plastic per patient per day, and this sector produces 8.5 percent of greenhouse gas emissions nationwide.
“Sustainable alternatives exist,” Stroetzel says, “but you have to painstakingly look for them; they are often not offered by the big manufacturers, and all of this takes way too much time [that] medical staff simply does not have during their hectic days.”
When Stroetzel spoke with medical staff in Germany, she found they were often frustrated by all of this waste, especially as they took care to avoid single-use plastic at home. Doctors in other countries share this frustration. In a recent poll, nine out of ten doctors in Germany said they’re aware of the urgency to find sustainable solutions in the health industry but don’t know how to achieve this goal.
After a year of researching more sustainable alternatives, Stroetzel founded a social enterprise startup called POP, short for Practice Without Plastic, together with IT expert Nicolai Niethe, to offer well-researched solutions. “Sustainable alternatives exist,” she says, “but you have to painstakingly look for them; they are often not offered by the big manufacturers, and all of this takes way too much time [that] medical staff simply does not have during their hectic days.”
In addition to reusable dentist cups, other good options for the heath care sector include washable N95 face masks and gloves made from nitrile, which waste less water and energy in their production. But Stroetzel admits that truly making a medical facility more sustainable is a complex task. “This includes negotiating with manufacturers who often package medical materials in double and triple layers of extra plastic.”
While initiatives such as Stroetzel’s provide much needed information, other experts reason that a wholesale rethinking of healthcare is needed. Voluntary action won’t be enough, and government should set the right example. Kari Nadeau, a Stanford physician who has spent 30 years researching the effects of environmental pollution on the immune system, and Kenneth Kizer, the former undersecretary for health in the U.S. Department of Veterans Affairs, wrote in JAMA last year that the medical industry and federal agencies that provide health care should be required to measure and make public their carbon footprints. “Government health systems do not disclose these data (and very rarely do private health care organizations), unlike more than 90% of the Standard & Poor’s top 500 companies and many nongovernment entities," they explained. "This could constitute a substantial step toward better equipping health professionals to confront climate change and other planetary health problems.”
Compared to the U.K., the U.S. healthcare industry lags behind in terms of measuring and managing its carbon footprint, and hospitals are the second highest energy user of any sector in the U.S.
Kizer and Nadeau look to the U.K. National Health Service (NHS), which created a Sustainable Development Unit in 2008 and began that year to conduct assessments of the NHS’s carbon footprint. The NHS also identified its biggest culprits: Of the 2019 footprint, with emissions totaling 25 megatons of carbon dioxide equivalent, 62 percent came from the supply chain, 24 percent from the direct delivery of care, 10 percent from staff commute and patient and visitor travel, and 4 percent from private health and care services commissioned by the NHS. From 1990 to 2019, the NHS has reduced its emission of carbon dioxide equivalents by 26 percent, mostly due to the switch to renewable energy for heat and power. Meanwhile, the NHS has encouraged health clinics in the U.K. to install wind generators or photovoltaics that convert light to electricity -- relatively quick ways to decarbonize buildings in the health sector.
Compared to the U.K., the U.S. healthcare industry lags behind in terms of measuring and managing its carbon footprint, and hospitals are the second highest energy user of any sector in the U.S. “We are already seeing patients with symptoms from climate change, such as worsened respiratory symptoms from increased wildfires and poor air quality in California,” write Thomas B. Newman, a pediatrist at the University of California, San Francisco, and UCSF clinical research coordinator Daisy Valdivieso. “Because of the enormous health threat posed by climate change, health professionals should mobilize support for climate mitigation and adaptation efforts.” They believe “the most direct place to start is to approach the low-lying fruit: reducing healthcare waste and overuse.”
In addition to resulting in waste, the plastic in hospitals ultimately harms patients, who may be even more vulnerable to the effects due to their health conditions. Microplastics have been detected in most humans, and on average, a human ingests five grams of microplastic per week. Newman and Valdivieso refer to the American Board of Internal Medicine's Choosing Wisely program as one of many initiatives that identify and publicize options for “safely doing less” as a strategy to reduce unnecessary healthcare practices, and in turn, reduce cost, resource use, and ultimately reduce medical harm.
A few U.S. clinics are pioneers in transitioning to clean energy sources. In Wisconsin, the nonprofit Gundersen Health network became the first hospital to cut its reliance on petroleum by switching to locally produced green energy in 2015, and it saved $1.2 million per year in the process. Kaiser Permanente eliminated its 800,000 ton carbon footprint through energy efficiency and purchasing carbon offsets, reaching a balance between carbon emissions and removing carbon from the atmosphere in 2020, the first U.S. health system to do so.
Cleveland Clinic has pledged to join Kaiser in becoming carbon neutral by 2027. Realizing that 80 percent of its 2008 carbon emissions came from electricity consumption, the Clinic started switching to renewable energy and installing solar panels, and it has invested in researching recyclable products and packaging. The Clinic’s sustainability report outlines several strategies for producing less waste, such as reusing cases for sterilizing instruments, cutting back on materials that can’t be recycled, and putting pressure on vendors to reduce product packaging.
The Charité Berlin, Europe’s biggest university hospital, has also announced its goal to become carbon neutral. Its sustainability managers have begun to identify the biggest carbon culprits in its operations. “We’ve already reduced CO2 emissions by 21 percent since 2016,” says Simon Batt-Nauerz, the director of infrastructure and sustainability.
The hospital still emits 100,000 tons of CO2 every year, as much as a city with 10,000 residents, but it’s making progress through ride share and bicycle programs for its staff of 20,000 employees, who can get their bikes repaired for free in one of the Charité-operated bike workshops. Another program targets doctors’ and nurses’ scrubs, which cause more than 200 tons of CO2 during manufacturing and cleaning. The staff is currently testing lighter, more sustainable scrubs made from recycled cellulose that is grown regionally and requires 80 percent less land use and 30 percent less water.

The Charité hospital in Berlin still emits 100,000 tons of CO2 every year, but it’s making progress through ride share and bicycle programs for its staff of 20,000 employees.
Wiebke Peitz | Specific to Charité
Anesthesiologist Susanne Koch spearheads sustainability efforts in anesthesiology at the Charité. She says that up to a third of hospital waste comes from surgery rooms. To reduce medical waste, she recommends what she calls the 5 Rs: Reduce, Reuse, Recycle, Rethink, Research. “In medicine, people don’t question the use of plastic because of safety concerns,” she says. “Nobody wants to be sued because something is reused. However, it is possible to reduce plastic and other materials safely.”
For instance, she says, typical surgery kits are single-use and contain more supplies than are actually needed, and the entire kit is routinely thrown out after the surgery. “Up to 20 percent of materials in a surgery room aren’t used but will be discarded,” Koch says. One solution could be smaller kits, she explains, and another would be to recycle the plastic. Another example is breathing tubes. “When they became scarce during the pandemic, studies showed that they can be used seven days instead of 24 hours without increased bacteria load when we change the filters regularly,” Koch says, and wonders, “What else can we reuse?”
In the Netherlands, TU Delft researchers Tim Horeman and Bart van Straten designed a method to melt down the blue polypropylene wrapping paper that keeps medical instruments sterile, so that the material can be turned it into new medical devices. Currently, more than a million kilos of the blue paper are used in Dutch hospitals every year. A growing number of Dutch hospitals are adopting this approach.
Another common practice that’s ripe for improvement is the use of a certain plastic, called PVC, in hospital equipment such as blood bags, tubes and masks. Because of its toxic components, PVC is almost never recycled in the U.S., but University of Michigan researchers Danielle Fagnani and Anne McNeil have discovered a chemical process that can break it down into material that could be incorporated back into production. This could be a step toward a circular economy “that accounts for resource inputs and emissions throughout a product’s life cycle, including extraction of raw materials, manufacturing, transport, use and reuse, and disposal,” as medical experts have proposed. “It’s a failure of humanity to have created these amazing materials which have improved our lives in many ways, but at the same time to be so shortsighted that we didn’t think about what to do with the waste,” McNeil said in a press release.
Susanne Koch puts it more succinctly: “What’s the point if we save patients while killing the planet?”
Meet Dr. Renee Wegrzyn, the first Director of President Biden's new health agency, ARPA-H
Today's podcast guest, Dr. Renee Wegrzyn, directs ARPA-H, a new agency formed last year to spearhead health innovations. Time will tell if ARPA-H will produce advances on the level of its fellow agency, DARPA.
In today’s podcast episode, I talk with Renee Wegrzyn, appointed by President Biden as the first director of a health agency created last year, the Advanced Research Projects Agency for Health, or ARPA-H. It’s inspired by DARPA, the agency that develops innovations for the Defense department and has been credited with hatching world-changing technologies such as ARPANET, which became the internet.
Time will tell if ARPA-H will lead to similar achievements in the realm of health. That’s what President Biden and Congress expect in return for funding ARPA-H at 2.5 billion dollars over three years.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
How will the agency figure out which projects to take on, especially with so many patient advocates for different diseases demanding moonshot funding for rapid progress?
I talked with Dr. Wegrzyn about the opportunities and challenges, what lessons ARPA-H is borrowing from Operation Warp Speed, how she decided on the first ARPA-H project that was announced recently, why a separate agency was needed instead of reforming HHS and the National Institutes of Health to be better at innovation, and how ARPA-H will make progress on disease prevention in addition to treatments for cancer, Alzheimer’s and diabetes, among many other health priorities.
Dr. Wegrzyn’s resume leaves no doubt of her suitability for this role. She was a program manager at DARPA where she focused on applying gene editing and synthetic biology to the goal of improving biosecurity. For her work there, she received the Superior Public Service Medal and, in case that wasn’t enough ARPA experience, she also worked at another ARPA that leads advanced projects in intelligence, called I-ARPA. Before that, she ran technical teams in the private sector working on gene therapies and disease diagnostics, among other areas. She has been a vice president of business development at Gingko Bioworks and headed innovation at Concentric by Gingko. Her training and education includes a PhD and undergraduate degree in applied biology from the Georgia Institute of Technology and she did her postdoc as an Alexander von Humboldt Fellow in Heidelberg, Germany.
Dr. Wegrzyn told me that she’s “in the hot seat.” The pressure is on for ARPA-H especially after the need and potential for health innovation was spot lit by the pandemic and the unprecedented speed of vaccine development. We'll soon find out if ARPA-H can produce gamechangers in health that are equivalent to DARPA’s creation of the internet.
Show links:
ARPA-H - https://arpa-h.gov/
Dr. Wegrzyn profile - https://arpa-h.gov/people/renee-wegrzyn/
Dr. Wegrzyn Twitter - https://twitter.com/rwegrzyn?lang=en
President Biden Announces Dr. Wegrzyn's appointment - https://www.whitehouse.gov/briefing-room/statement...
Leaps.org coverage of ARPA-H - https://leaps.org/arpa/
ARPA-H program for joints to heal themselves - https://arpa-h.gov/news/nitro/ -
ARPA-H virtual talent search - https://arpa-h.gov/news/aco-talent-search/

Dr. Renee Wegrzyn was appointed director of ARPA-H last October.
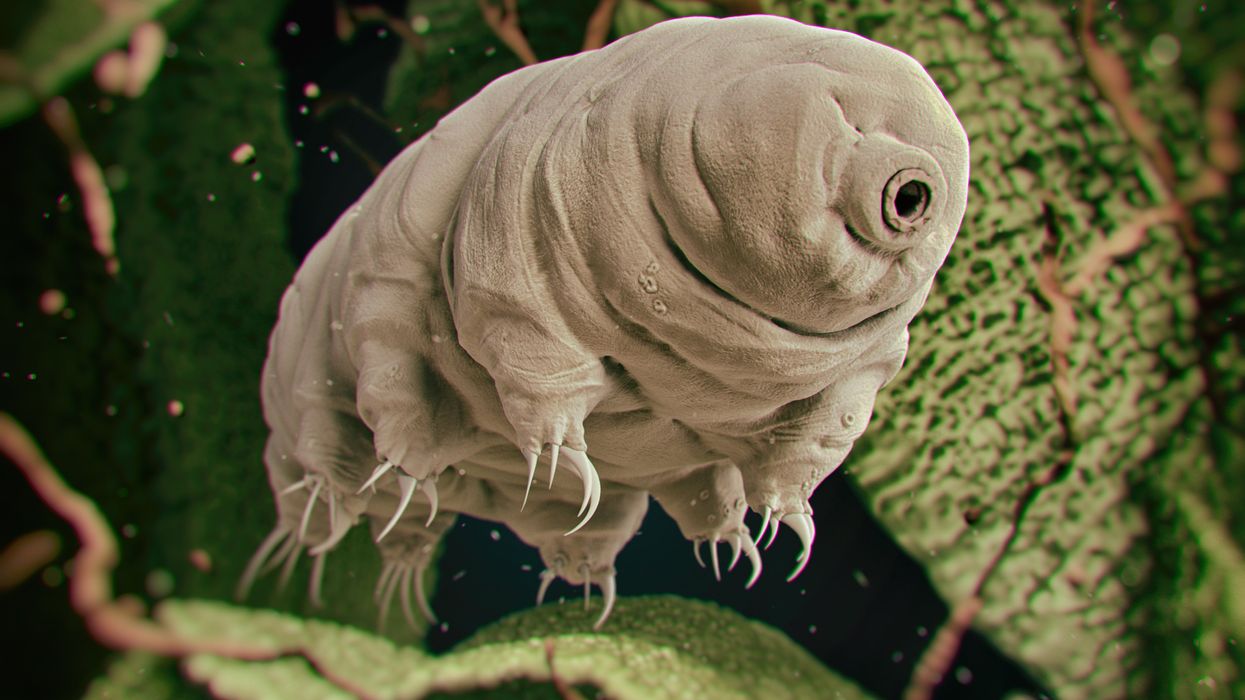
Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.