These technologies may help more animals and plants survive climate change

As the climate changes, the ripples will reach everywhere. Better data is needed for both plants and animals, and scientists are looking for genes that could allow crops to survive.
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are making us more vulnerable to infectious diseases by land and by sea - and how scientists are working on solutions.
Along the west coast of South Florida and the Keys, Florida Bay is a nursery for young Caribbean spiny lobsters, a favorite local delicacy. Growing up in small shallow basins, they are especially vulnerable to warmer, more saline water. Climate change has brought tidal floods, bleached coral reefs and toxic algal blooms to the state, and since the 1990s, the population of the Caribbean spiny lobster has dropped some 20 percent, diminishing an important food for snapper, grouper, and herons, as well as people. In 1999, marine ecologist Donald Behringer discovered the first known virus among lobsters, Panulirus argus virus—about a quarter of juveniles die from it before they mature.
“When the water is warm PaV1 progresses much more quickly,” says Behringer, who is based at the Emerging Pathogens Institute at the University of Florida in Gainesville.
Caribbean spiny lobsters are only one example of many species that are struggling in the era of climate change, both at sea and on land. As the oceans heat up, absorbing greenhouse gases and growing more acidic, marine diseases are emerging at an accelerated rate. Marine creatures are migrating to new places, and carrying pathogens with them. The latest grim report in the journal Science, states that if global warming continues at the current rate, the extinction of marine species will rival the Permian–Triassic extinction, sometimes called the “Great Dying,” when volcanoes poisoned the air and wiped out as much as 90 percent of all marine life 252 million years ago.
Similarly, on land, climate change has exposed wildlife, trees and crops to new or more virulent pathogens. Warming environments allow fungi, bacteria, viruses and infectious worms to proliferate in new species and locations or become more virulent. One paper modeling records of nearly 1,400 wildlife species projects that parasites will double by 2070 in the far north and in high-altitude places. Right now, we are seeing the effects most clearly on the fringes—along the coasts, up north and high in the mountains—but as the climate continues changing, the ripples will reach everywhere.
Few species are spared
On the Hawaiian Islands, mosquitoes are killing more songbirds. The dusky gray akikiki of Kauai and the chartreuse-yellow kiwikiu of Maui could vanish in two years, under assault from mosquitoes bearing avian malaria, according to a University of Hawaiʻi 2022 report. Previously, the birds could escape infection by roosting high in the cold mountains, where the pests couldn’t thrive, but climate change expanded the range of the mosquito and narrowed theirs.
Likewise, as more midge larvae survive over warm winters and breed better during drier summers, they bite more white-tailed deer, spreading often-fatal epizootic hemorrhagic disease. Especially in northern regions of the globe, climate change brings the threat of midges carrying blue tongue disease, a virus, to sheep and other animals. Tick-borne diseases like encephalitis and Lyme disease may become a greater threat to animals and perhaps humans.
"If you put all your eggs in one basket and then a pest comes a long, then you are more vulnerable to those risks," says Mehroad Ehsani, managing director of the food initiative in Africa for the Rockefeller Foundation. "Research is needed on resilient, climate smart, regenerative agriculture."
In the “thermal mismatch” theory of wildlife disease, cold-adapted species are at greater risk when their habitats warm, and warm-adapted species suffer when their habitats cool. Mammals can adjust their body temperature to adapt to some extent. Amphibians, fish and insects that cannot regulate body temperatures may be at greater risk. Many scientists see amphibians, especially, as canaries in the coalmine, signaling toxicity.
Early melting ice can foster disease. Climate models predict that the spring thaw will come ever-earlier in the lakes of the French Pyrenees, for instance, which traditionally stayed frozen for up to half the year. The tadpoles of the midwife toad live under the ice, where they are often infected with amphibian chytrid fungus. When a seven-year study tracked the virus in three species of amphibians in Pyrenees’s Lac Arlet, the research team found that, the earlier the spring thaw arrived, the more infection rates rose in common toads— , while remaining high among the midwife toads. But the team made another sad discovery: with early thaws, the common frog, which was thought to be free of the disease in Europe, also became infected with the fungus and died in large numbers.
Changing habitats affect animal behavior. Normally, spiny lobsters rely on chemical cues to avoid predators and sick lobsters. New conditions may be hampering their ability to “social distance”—which may help PaV1 spread, Behringer’s research suggests. Migration brings other risks. In April 2022, an international team led by scientists at Georgetown University announced the first comprehensive overview, published in the journal Nature, of how wild mammals under pressure from a changing climate may mingle with new populations and species—giving viruses a deadly opportunity to jump between hosts. Droughts, for example, will push animals to congregate at the few places where water remains.
Plants face threats also. At the timberline of the cold, windy, snowy mountains of the U.S. west, whitebark pine forests are facing a double threat, from white pine blister rust, a fungal disease, and multiplying pine beetles. “If we do nothing, we will lose the species,” says Robert Keane, a research ecologist for the U.S. Forest Service, based in Missoula, Montana. That would be a huge shift, he explains: “It’s a keystone species. There are over 110 animals that depend on it, many insects, and hundreds of plants.” In the past, beetle larvae would take two years to complete their lifecycle, and many died in frost. “With climate change, we're seeing more and more beetles survive, and sometimes the beetle can complete its lifecycle in one year,” he says.
Quintessential crops are under threat too
As some pathogens move north and new ones develop, they pose novel threats to the crops humans depend upon. This is already happening to wheat, coffee, bananas and maize.
Breeding against wheat stem rust, a fungus long linked to famine, was a key success in the mid-20th century Green Revolution, which brought higher yields around the world. In 2013, wheat stem rust reemerged in Germany after decades of absence. It ravaged both bread and durum wheat in Sicily in 2016 and has spread as far as England and Ireland. Wheat blast disease, caused by a different fungus, appeared in Bangladesh in 2016, and spread to India, the world’s second largest producer of wheat.
Insects, moths, worms, and coffee leaf rust—a fungus now found in all coffee-growing countries—threaten the livelihoods of millions of people who grow coffee, as well as everybody’s cup of joe. More heat, more intense rain, and higher humidity have allowed coffee leaf rust to cycle more rapidly. It has grown exponentially, overcoming the agricultural chemicals that once kept it under control.
To identify new diseases and fine-tune crops for resistance, scientists are increasingly relying on genomic tools.
Tar spot, a fungus native to Latin America that can cut corn production in half, has emerged in highland areas of Central Mexico and parts of the U.S.. Meanwhile, maize lethal necrosis disease has spread to multiple countries in Africa, notes Mehrdad Ehsani, Managing Director for the Food Initiative in Africa of the Rockefeller Foundation. The Cavendish banana, which most people eat today, was bred to be resistant to the fungus Panama 1. Now a new fungus, Panama 4, has emerged on every continent–including areas of Latin America that rely on the Cavendish for their income, reported a recent story in the Guardian. New threats are poised to emerge. Potato growers in the Andes Mountains have been shielded from disease because of colder weather at high altitude, but temperature fluxes and warming weather are expected to make this crop vulnerable to potato blight, found plant pathologist Erica Goss, at the Emerging Pathogens Institute.
Science seeks solutions
To protect food supplies in the era of climate change, scientists are calling for integrated global surveillance systems for crop disease outbreaks. “You can imagine that a new crop variety that is drought-tolerant could be susceptible to a pathogen that previous varieties had some resistance against,” Goss says. “Or a country suffers from a calamitous weather event, has to import seed from another country, and that seed is contaminated with a new pathogen or more virulent strain of an existing pathogen.” Researchers at the John Innes Center in Norwich and Aarhus University in Denmark have established ways to monitor wheat rust, for example.
Better data is essential, for both plants and animals. Historically, models of climate change predicted effects on plant pathogens based on mean temperatures, and scientists tracked plant responses to constant temperatures, explains Goss. “There is a need for more realistic tests of the effects of changing temperatures, particularly changes in daily high and low temperatures on pathogens,” she says.
To identify new diseases and fine-tune crops for resistance, scientists are increasingly relying on genomic tools. Goss suggests factoring the impact of climate change into those tools. Genomic efforts to select soft red winter wheat that is resistant to Fusarium head blight (FHB), a fungus that plagues farmers in the Southeastern U.S., have had early success. But temperature changes introduce a new factor.
A fundamental solution would be to bring back diversification in farming, says Ehsani. Thousands of plant species are edible, yet we rely on a handful. Wild relatives of domesticated crops are a store of possibly useful genes that may confer resistance to disease. The same is true for livestock. “If you put all your eggs in one basket and then a pest comes along, then you are more vulnerable to those risks. Research is needed on resilient, climate smart, regenerative agriculture,” Ehsani says.
Jonathan Sleeman, director of the U.S. Geological Survey National Wildlife Health Center, has called for data on wildlife health to be systematically collected and integrated with climate and other variables because more comprehensive data will result in better preventive action. “We have focused on detecting diseases,” he says, but a more holistic strategy would apply human public health concepts to assuring animal wellbeing. (For example, one study asked experts to draw a diagram of relationships of all the factors affecting the health of a particular group of caribou.) We must not take the health of plants and animals for granted, because their vulnerability inevitably affects us too, Sleeman says. “We need to improve the resilience of wildlife populations so they can withstand the impact of climate change.”
DNA- and RNA-based electronic implants may revolutionize healthcare
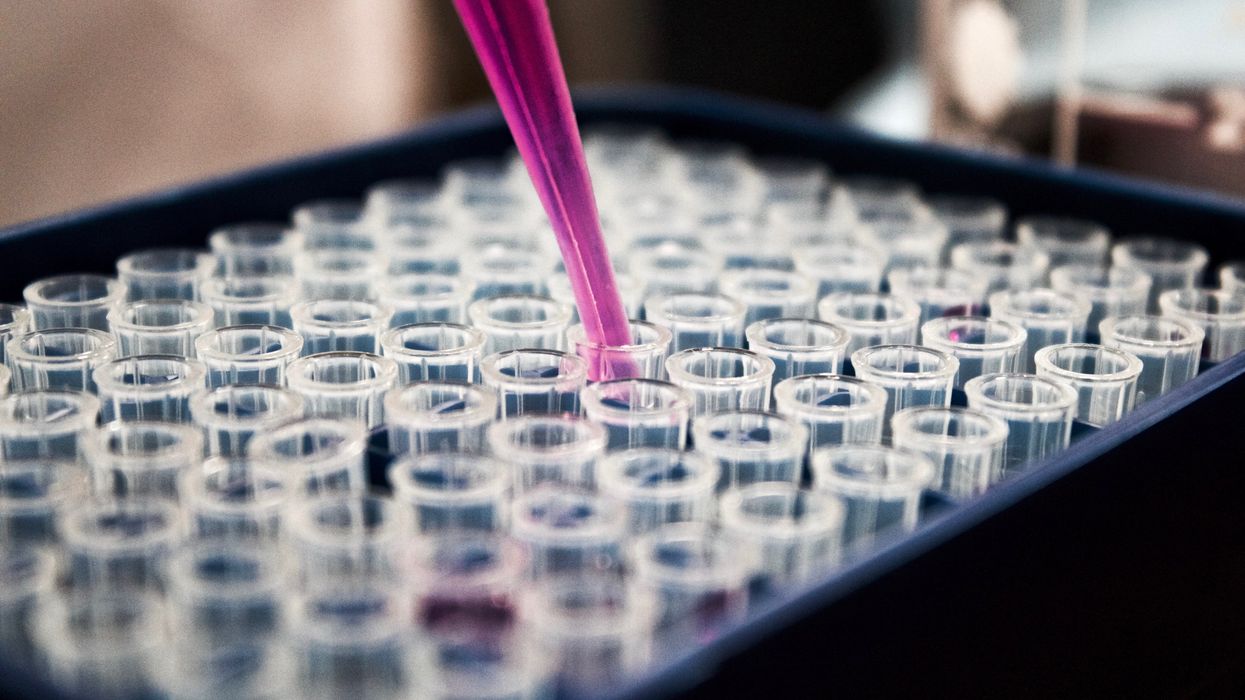
The test tubes contain tiny DNA/enzyme-based circuits, which comprise TRUMPET, a new type of electronic device, smaller than a cell.
Implantable electronic devices can significantly improve patients’ quality of life. A pacemaker can encourage the heart to beat more regularly. A neural implant, usually placed at the back of the skull, can help brain function and encourage higher neural activity. Current research on neural implants finds them helpful to patients with Parkinson’s disease, vision loss, hearing loss, and other nerve damage problems. Several of these implants, such as Elon Musk’s Neuralink, have already been approved by the FDA for human use.
Yet, pacemakers, neural implants, and other such electronic devices are not without problems. They require constant electricity, limited through batteries that need replacements. They also cause scarring. “The problem with doing this with electronics is that scar tissue forms,” explains Kate Adamala, an assistant professor of cell biology at the University of Minnesota Twin Cities. “Anytime you have something hard interacting with something soft [like muscle, skin, or tissue], the soft thing will scar. That's why there are no long-term neural implants right now.” To overcome these challenges, scientists are turning to biocomputing processes that use organic materials like DNA and RNA. Other promised benefits include “diagnostics and possibly therapeutic action, operating as nanorobots in living organisms,” writes Evgeny Katz, a professor of bioelectronics at Clarkson University, in his book DNA- And RNA-Based Computing Systems.
While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output.
Adamala’s research focuses on developing such biocomputing systems using DNA, RNA, proteins, and lipids. Using these molecules in the biocomputing systems allows the latter to be biocompatible with the human body, resulting in a natural healing process. In a recent Nature Communications study, Adamala and her team created a new biocomputing platform called TRUMPET (Transcriptional RNA Universal Multi-Purpose GatE PlaTform) which acts like a DNA-powered computer chip. “These biological systems can heal if you design them correctly,” adds Adamala. “So you can imagine a computer that will eventually heal itself.”
The basics of biocomputing
Biocomputing and regular computing have many similarities. Like regular computing, biocomputing works by running information through a series of gates, usually logic gates. A logic gate works as a fork in the road for an electronic circuit. The input will travel one way or another, giving two different outputs. An example logic gate is the AND gate, which has two inputs (A and B) and two different results. If both A and B are 1, the AND gate output will be 1. If only A is 1 and B is 0, the output will be 0 and vice versa. If both A and B are 0, the result will be 0. While a computer gives these inputs in binary code or "bits," such as a 0 or 1, biocomputing uses DNA strands as inputs, whether double or single-stranded, and often uses fluorescent RNA as an output. In this case, the DNA enters the logic gate as a single or double strand.
If the DNA is double-stranded, the system “digests” the DNA or destroys it, which results in non-fluorescence or “0” output. Conversely, if the DNA is single-stranded, it won’t be digested and instead will be copied by several enzymes in the biocomputing system, resulting in fluorescent RNA or a “1” output. And the output for this type of binary system can be expanded beyond fluorescence or not. For example, a “1” output might be the production of the enzyme insulin, while a “0” may be that no insulin is produced. “This kind of synergy between biology and computation is the essence of biocomputing,” says Stephanie Forrest, a professor and the director of the Biodesign Center for Biocomputing, Security and Society at Arizona State University.
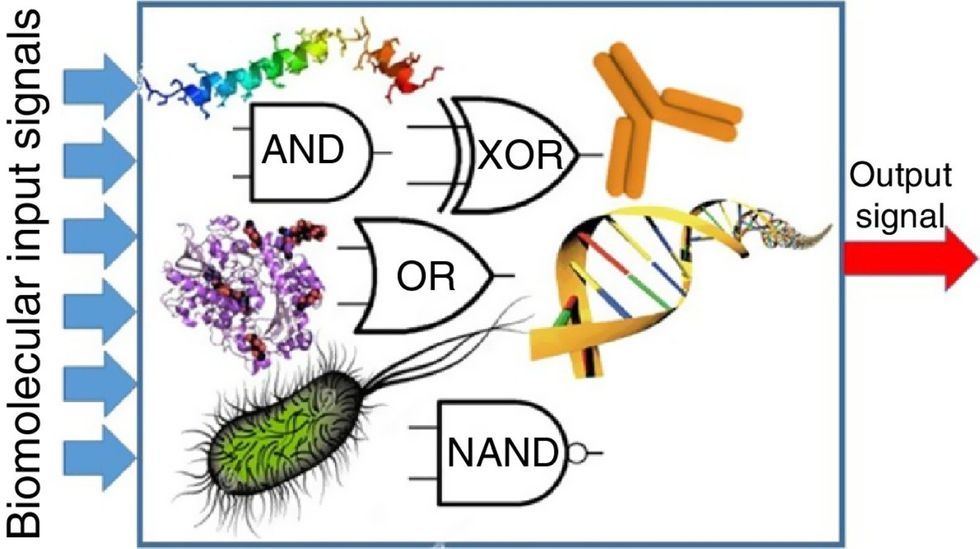
Biocomputing circles are made of DNA, RNA, proteins and even bacteria.
Evgeny Katz
The TRUMPET’s promise
Depending on whether the biocomputing system is placed directly inside a cell within the human body, or run in a test-tube, different environmental factors play a role. When an output is produced inside a cell, the cell's natural processes can amplify this output (for example, a specific protein or DNA strand), creating a solid signal. However, these cells can also be very leaky. “You want the cells to do the thing you ask them to do before they finish whatever their businesses, which is to grow, replicate, metabolize,” Adamala explains. “However, often the gate may be triggered without the right inputs, creating a false positive signal. So that's why natural logic gates are often leaky." While biocomputing outside a cell in a test tube can allow for tighter control over the logic gates, the outputs or signals cannot be amplified by a cell and are less potent.
TRUMPET, which is smaller than a cell, taps into both cellular and non-cellular biocomputing benefits. “At its core, it is a nonliving logic gate system,” Adamala states, “It's a DNA-based logic gate system. But because we use enzymes, and the readout is enzymatic [where an enzyme replicates the fluorescent RNA], we end up with signal amplification." This readout means that the output from the TRUMPET system, a fluorescent RNA strand, can be replicated by nearby enzymes in the platform, making the light signal stronger. "So it combines the best of both worlds,” Adamala adds.
These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body.
The TRUMPET biocomputing process is relatively straightforward. “If the DNA [input] shows up as single-stranded, it will not be digested [by the logic gate], and you get this nice fluorescent output as the RNA is made from the single-stranded DNA, and that's a 1,” Adamala explains. "And if the DNA input is double-stranded, it gets digested by the enzymes in the logic gate, and there is no RNA created from the DNA, so there is no fluorescence, and the output is 0." On the story's leading image above, if the tube is "lit" with a purple color, that is a binary 1 signal for computing. If it's "off" it is a 0.
While still in research, TRUMPET and other biocomputing systems promise significant benefits to personalized healthcare and medicine. These organic-based systems could detect cancer cells or low insulin levels inside a patient’s body. The study’s lead author and graduate student Judee Sharon is already beginning to research TRUMPET's ability for earlier cancer diagnoses. Because the inputs for TRUMPET are single or double-stranded DNA, any mutated or cancerous DNA could theoretically be detected from the platform through the biocomputing process. Theoretically, devices like TRUMPET could be used to detect cancer and other diseases earlier.
Adamala sees TRUMPET not only as a detection system but also as a potential cancer drug delivery system. “Ideally, you would like the drug only to turn on when it senses the presence of a cancer cell. And that's how we use the logic gates, which work in response to inputs like cancerous DNA. Then the output can be the production of a small molecule or the release of a small molecule that can then go and kill what needs killing, in this case, a cancer cell. So we would like to develop applications that use this technology to control the logic gate response of a drug’s delivery to a cell.”
Although platforms like TRUMPET are making progress, a lot more work must be done before they can be used commercially. “The process of translating mechanisms and architecture from biology to computing and vice versa is still an art rather than a science,” says Forrest. “It requires deep computer science and biology knowledge,” she adds. “Some people have compared interdisciplinary science to fusion restaurants—not all combinations are successful, but when they are, the results are remarkable.”
Crickets are low on fat, high on protein, and can be farmed sustainably. They are also crunchy.
In today’s podcast episode, Leaps.org Deputy Editor Lina Zeldovich speaks about the health and ecological benefits of farming crickets for human consumption with Bicky Nguyen, who joins Lina from Vietnam. Bicky and her business partner Nam Dang operate an insect farm named CricketOne. Motivated by the idea of sustainable and healthy protein production, they started their unconventional endeavor a few years ago, despite numerous naysayers who didn’t believe that humans would ever consider munching on bugs.
Yet, making creepy crawlers part of our diet offers many health and planetary advantages. Food production needs to match the rise in global population, estimated to reach 10 billion by 2050. One challenge is that some of our current practices are inefficient, polluting and wasteful. According to nonprofit EarthSave.org, it takes 2,500 gallons of water, 12 pounds of grain, 35 pounds of topsoil and the energy equivalent of one gallon of gasoline to produce one pound of feedlot beef, although exact statistics vary between sources.
Meanwhile, insects are easy to grow, high on protein and low on fat. When roasted with salt, they make crunchy snacks. When chopped up, they transform into delicious pâtes, says Bicky, who invents her own cricket recipes and serves them at industry and public events. Maybe that’s why some research predicts that edible insects market may grow to almost $10 billion by 2030. Tune in for a delectable chat on this alternative and sustainable protein.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
Further reading:
More info on Bicky Nguyen
https://yseali.fulbright.edu.vn/en/faculty/bicky-n...
The environmental footprint of beef production
https://www.earthsave.org/environment.htm
https://www.watercalculator.org/news/articles/beef-king-big-water-footprints/
https://www.frontiersin.org/articles/10.3389/fsufs.2019.00005/full
https://ourworldindata.org/carbon-footprint-food-methane
Insect farming as a source of sustainable protein
https://www.insectgourmet.com/insect-farming-growing-bugs-for-protein/
https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/insect-farming
Cricket flour is taking the world by storm
https://www.cricketflours.com/
https://talk-commerce.com/blog/what-brands-use-cricket-flour-and-why/

Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.