Is Carbon Dioxide the New Black? Yes, If These Fabric-Designing Scientists Have Their Way

The entrepreneurs Tawfiq Nasr Allah (left, standing) and Benoit Illy (right, sitting down) at Fairbrics' lab in Clichy, France.
Each year the world releases around 33 billion tons of carbon dioxide into the atmosphere. What if we could use this waste carbon dioxide to make shirts, dresses and hats? It sounds unbelievable. But two innovators are trying to tackle climate change in this truly unique way.
Chemist Tawfiq Nasr Allah set up Fairbrics with material scientist Benoît Illy in 2019. They're using waste carbon dioxide from industrial fumes as a raw material to create polyester, identical to the everyday polyester we use now. They want to take a new and very different approach to make the fashion industry more sustainable.
The Dark Side of Fast Fashion
The fashion industry is responsible for around 4% of global emissions. In a 2015 report, the MIT Materials Systems Laboratory predicted that the global impact of polyester fabric will grow from around 880 billion kg of CO2 in 2015 to 1.5 trillion kg of CO2 by 2030.
Professor Greg Peters, an expert in environmental science and sustainability, highlights the wide-ranging difficulties caused by the production of polyester. "Because it is made from petrochemical crude oil there is no real limit on how much polyester can be produced...You have to consider the ecological damage (oil spills, fracking etc.) caused by the oil and gas industry."
Many big-name brands have pledged to become carbon neutral by 2050. But nothing has really changed in the way polyester is produced.
Some companies are recycling plastic bottles into polyester. The plastic is melted into ultra-fine strands and then spun to create polyester. However, only a limited number of bottles are available. New materials must be added because of the amount of plastic degradation that takes place. Ultimately, recycling accounts for only a small percentage of the total amount of polyester produced.
Nasr Allah and Illy hope they can offer the solution the fashion industry is looking for. They are not just reducing the carbon emissions that are conventionally produced by making polyester. Their process actually goes much further. It's carbon negative and works by using up emissions from other industries.
"In a sense we imitate what nature does so well: plants capture CO2 and turn it into natural fibers using sunlight, we capture CO2 and turn it into synthetic fibers using electricity."
Experts in the field see a lot of promise. Dr Phil de Luna is an expert in carbon valorization -- the process of converting carbon dioxide into high-value chemicals. He leads a $57-million research program developing the technology to decarbonize Canada.
"I think the approach is great," he says. "Being able to take CO2 and then convert it into polymers or polyester is an excellent way to think about utilizing waste emissions and replacing fossil fuel-based materials. That is overall a net negative as compared to making polyester from fossil fuels."
From Harmful Waste to Useful Raw Material
It all started with Nasr Allah's academic research, primarily at the French Alternative Energies and Atomic Energy Commission (CEA). He spent almost 5 years investigating CO2 valorization. In essence, this involves breaking the bonds between the carbon and oxygen atoms in CO2 to create bonds with other elements.
Recycling carbon dioxide in this way requires extremely high temperatures and pressures. Catalysts are needed to break the strong bonds between the atoms. However, these are toxic, volatile and quickly lose their effectiveness over time. So, directly converting carbon dioxide into the raw material for making polyester fibers is very difficult.
Nasr Allah developed a process involving multiple simpler stages. His innovative approach involves converting carbon dioxide to intermediate chemicals. These chemicals can then be transformed into the raw material which is used in the production of polyester. After many experiments, Nasr Allah developed new processes and new catalysts that worked more effectively.
"We use a catalyst to transform CO2 into the chemicals that are used for polyester manufacturing," Illy says. "In a sense we imitate what nature does so well: plants capture CO2 and turn it into natural fibers using sunlight, we capture CO2 and turn it into synthetic fibers using electricity."
The Challenges Ahead
Nasr Allah met material scientist Illy through Entrepreneur First, a programme which pairs individuals looking to form technical start-ups. Together they set up Fairbrics and worked on converting Nasr Allah's lab findings into commercial applications and industrial success.
"The main challenge we faced was to scale up the process," Illy reveals. "[It had to be] consistent and safe to be carried out by a trained technician, not a specialist PhD as was the case in the beginning."
They recruited a team of scientists to help them develop a more effective and robust manufacturing process. Together, the team gained a more detailed theoretical understanding about what was happening at each stage of the chemical reactions. Eventually, they were able to fine tune the process and produce consistent batches of polyester.
They're making significant progress. They've produced their first samples and signed their first commercial contract to make polyester, which will then be both fabricated into clothes and sold by partner companies.
Currently, one of the largest challenges is financial. "We need to raise a fair amount to buy the equipment we need to produce at a large scale," Illy explains.
How to Power the Process?
At the moment, their main scientific focus is getting the process working reliably so they can begin commercialization. In order to remain sustainable and economically viable once they start producing polyester on a large scale, they need to consider the amount of energy they use for carbon valorization and the emissions they produce.
The more they optimize the way their catalyst works, the easier it will be to transform the CO2. The whole process can then become more cost effective and energy efficient.
De Luna explains: "My concern is...whether their process will be economical at scale. The problem is the energy cost to take carbon dioxide and transform it into these other products and that's where the science and innovation has to happen. [Whether they can scale up economically] depends on the performance of their catalyst."
They don't just need to think about the amount of energy they use to produce polyester; they also have to consider where this energy comes from.
"They need access to cheap renewable energy," De Luna says, "...so they're not using or emitting CO2 to do the conversion." If the energy they use to transform CO2 into polyester actually ends up producing more CO2, this will end up cancelling out their positive environmental impact.
Based in France, they're well located to address this issue. France has a clean electricity system, with only about 10% of their electric power coming from fossil fuels due to their reliance on nuclear energy and renewables.
Where Do They Get the Carbon Dioxide?
As they scale up, they also need to be able to access a source of CO2. They intend to obtain this from the steel industry, the cement industry, and hydrogen production.
The technology to purify and capture waste carbon dioxide from these industries is available on a large scale. However, there are only around 20 commercial operations in the world. The high cost of carbon capture means that development continues to be slow. There are a growing number of startups capturing carbon dioxide straight from the air, but this is even more costly.
One major problem is that storing captured carbon dioxide is expensive. "There are somewhat limited options for permanently storing captured CO2, so innovations like this are important,'' says T. Reed Miller, a researcher at the Yale University Center for Industrial Ecology.
Illy says: "The challenge is now to decrease the cost [of carbon capture]. By using CO2 as a raw material, we can try to increase the number of industries that capture CO2. Our goal is to turn CO2 from a waste into a valuable product."
Beyond Fashion
For Nasr Allah and Illy, fashion is just the beginning. There are many markets they can potentially break into. Next, they hope to use the polyester they've created in the packaging industry. Today, a lot of polyester is consumed to make bottles and jars. Illy believes that eventually they can produce many different chemicals from CO2. These chemicals could then be used to make paints, adhesives, and even plastics.
The Fairbrics scientists are providing a vital alternative to fossil fuels and showcasing the real potential of carbon dioxide to become a worthy resource instead of a harmful polluter.
Illy believes they can make a real difference through innovation: "We can have a significant impact in reducing climate change."
Inside Scoop: How a DARPA Scientist Helped Usher in a Game-Changing Covid Treatment
Amy Jenkins is a program manager for the Defense Advanced Research Projects Agency's Biological Technologies Office, which runs a project called the Pandemic Prevention Platform.
Amy Jenkins was in her office at DARPA, a research and development agency within the Department of Defense, when she first heard about a respiratory illness plaguing the Chinese city of Wuhan. Because she's a program manager for DARPA's Biological Technologies Office, her colleagues started stopping by. "It's really unusual, isn't it?" they would say.
At the time, China had a few dozen cases of what we now call COVID-19. "We should maybe keep an eye on that," she thought.
Early in 2020, still just keeping watch, she was visiting researchers working on DARPA's Pandemic Prevention Platform (P3), a project to develop treatments for "any known or previously unknown infectious threat," within 60 days of its appearance. "We looked at each other and said, 'Should we be doing something?'" she says.
For projects like P3, groups of scientists—often at universities and private companies—compete for DARPA contracts, and program managers like Jenkins oversee the work. Those that won the P3 bid included scientists at AbCellera Biologics, Inc., AstraZeneca, Duke University, and Vanderbilt University.
At the time Jenkins was talking to the P3 performers, though, they didn't have evidence of community transmission. "We would have to cross that bar before we considered doing anything," she says.
The world soon leapt far over that bar. By the time Jenkins and her team decided P3 should be doing something—with their real work beginning in late February--it was too late to prevent this pandemic. But she could help P3 dig into the chemical foundations of COVID-19's malfeasance, and cut off its roots. That work represents, in fact, her roots.
In late February 2020, DARPA received a single blood sample from a recovered COVID-19 patient, in which P3 researchers could go fishing for antibodies. The day it arrived, Jenkins's stomach roiled. "We get one shot," she thought.
Fighting the Smallest Enemies
Jenkins, who's in her early 40s, first got into germs the way many 90s kids did: by reading The Hot Zone, a novel about a hemorrhagic fever gone rogue. It wasn't exactly the disintegrating organs that hooked her. It was the idea that "these very pathogens that we can't even see can make us so sick and bring us to our knees," she says. Reading about scientists facing down deadly disease, she wondered, "How do these things make you so sick?"
She chased that question in college, majoring in both biomolecular science and chemistry, and later became an antibody expert. Antibodies are proteins that hook to a pathogen to block it from attaching to your cells, or tag it for destruction by the rest of the immune system. Soon, she jumped on the "monoclonal antibodies" train—developing synthetic versions of these natural defenses, which doctors can give to people to help them battle an early-stage infection, and even to prevent an infection from taking root after an exposure.
Jenkins likens the antibody treatments to the old aphorism about fishing: Vaccines teach your body how to fish, but antibodies simply give your body the pesca-fare. While that, as the saying goes, won't feed you for a lifetime, it will last a few weeks or months. Monoclonal antibodies thus are a promising preventative option in the immediate short-term when a vaccine hasn't yet been given (or hasn't had time to produce an immune response), as well as an important treatment weapon in the current fight. After former president Donald Trump contracted COVID-19, he received a monoclonal antibody treatment from biotech company Regeneron.
As for Jenkins, she started working as a DARPA Biological Technologies Office contractor soon after completing her postdoc. But it was a suit job, not a labcoat job. And suit jobs, at first, left Jenkins conflicted, worried about being bored. She'd give it a year, she thought. But the year expired, and bored she was not. Around five years later, in June 2019, the agency hired her to manage several of the office's programs. A year into that gig, the world was months into a pandemic.
The Pandemic Pivot
At DARPA, Jenkins inherited five programs, including P3. P3 works by taking blood from recovered people, fishing out their antibodies, identifying the most effective ones, and then figuring out how to manufacture them fast. Back then, P3 existed to help with nebulous, future outbreaks: Pandemic X. Not this pandemic. "I did not have a crystal ball," she says, "but I will say that all of us in the infectious diseases and public-health realm knew that the next pandemic was coming."
Three days after a January 2020 meeting with P3 researchers, COVID-19 appeared in Seattle, then began whipping through communities. The time had come for P3 teams to swivel. "We had done this," she says. "We had practiced this before." But would their methods stand up to something unknown, racing through the global population? "The big anxiety was, 'Wow, this was real,'" says Jenkins.
While facing down that realness, Jenkins was also managing other projects. In one called PREPARE, groups develop "medical countermeasures" that modulate a person's genetic code to boost their bodies' responses to threats. Another project, NOW, envisions shipping-container-sized factories that can make thousands of vaccine doses in days. And then there's Prometheus—which means "forethought" in Greek, and is the name of the god who stole fire and gave it to humans. Wrapping up as COVID ramped up, Prometheus aimed to identify people who are contagious—with whatever—before they start coughing, and even if they never do.
All of DARPA's projects focus on developing early-stage technology, passing it off to other agencies or industry to put it into operation. The orientation toward a specific goal appealed to Jenkins, as a contrast to academia. "You go down a rabbit hole for years at a time sometimes, chasing some concept you found interesting in the lab," she says. That's good for the human pursuit of knowledge, and leads to later applications, but DARPA wants a practical prototype—stat.
"Dual-Use" Technologies
That desire, though, and the fact that DARPA is a defense agency, present philosophical complications. "Bioethics in the national-security context turns all the dials up to 10+," says Jonathan Moreno, a medical ethicist at the University of Pennsylvania.
While developing antibody treatments to stem a pandemic seems straightforwardly good, all biological research—especially that backed by military money—requires evaluating potential knock-on applications, even those that might come from outside the entity that did the developing. As Moreno put it, "Albert Einstein wasn't thinking about blowing up Hiroshima." Particularly sensitive are so-called "dual-use" technologies—those tools that could be used for both benign and nefarious purposes, or are of interest to both the civilian and military worlds.
Moreno takes Prometheus itself as an example of "dual-use" technology. "Think about somebody wearing a suicide vest. Instead of a suicide vest, make them extremely contagious with something. The flu plus Ebola," he says. "Send them someplace, a sensitive environment. We would like to be able to defend against that"—not just tell whether Uncle Fred is bringing asymptomatic COVID home for Christmas. Prometheus, Jenkins says, had safety in mind from the get-go, and required contenders to "develop a risk mitigation plan" and "detail their strategy for appropriate control of information."
To look at a different program, if you can modulate genes to help healing, you probably know something (or know someone else could infer something) about how to hinder healing. Those sorts of risks are why PREPARE researchers got their own "ethical, legal, and social implications" panel, which meets quarterly "to ensure that we are performing all research and publications in a safe and ethical manner," says Jenkins.
DARPA as a whole, Moreno says, is institutionally sensitive to bioethics. The agency has ethics panels, and funded a 2014 National Academies assessment of how to address the "ethical, legal, and societal issues" around technology that has military relevance. "In the cases of biotechnologies where some of that research brushes up against what could legitimately be considered dual-use, that in itself justifies our investment," says Jenkins. "DARPA deliberately focuses on safety and countermeasures against potentially dangerous technologies, and we structure our programs to be transparent, safe, and legal."
Going Fishing
In late February 2020, DARPA received a single blood sample from a recovered COVID-19 patient, in which P3 researchers could go fishing for antibodies. The day it arrived, Jenkins's stomach roiled. "We get one shot," she thought.
As scientists from the P3-funded AbCellera went through the processes they'd practiced, Jenkins managed their work, tracking progress and relaying results. Soon, the team had isolated a suitable protein: bamlanivimab. It attaches to and blocks off the infamous spike proteins on SARS-CoV-2—those sticky suction-cups in illustrations. Partnering with Eli Lilly in a manufacturing agreement, the biotech company brought it to clinical trials in May, just a few months after its work on the deadly pathogen began, after much of the planet became a hot zone.
On November 10—Jenkins's favorite day at the (home) office—the FDA provided Eli Lilly emergency use authorization for bamlanivimab. But she's only mutedly screaming (with joy) inside her heart. "This pandemic isn't 'one morning we're going to wake up and it's all over,'" she says. When it is over, she and her colleagues plan to celebrate their promethean work. "I'm hoping to be able to do it in person," she says. "Until then, I have not taken a breath."
Everyone Should Hear My COVID Vaccine Experience
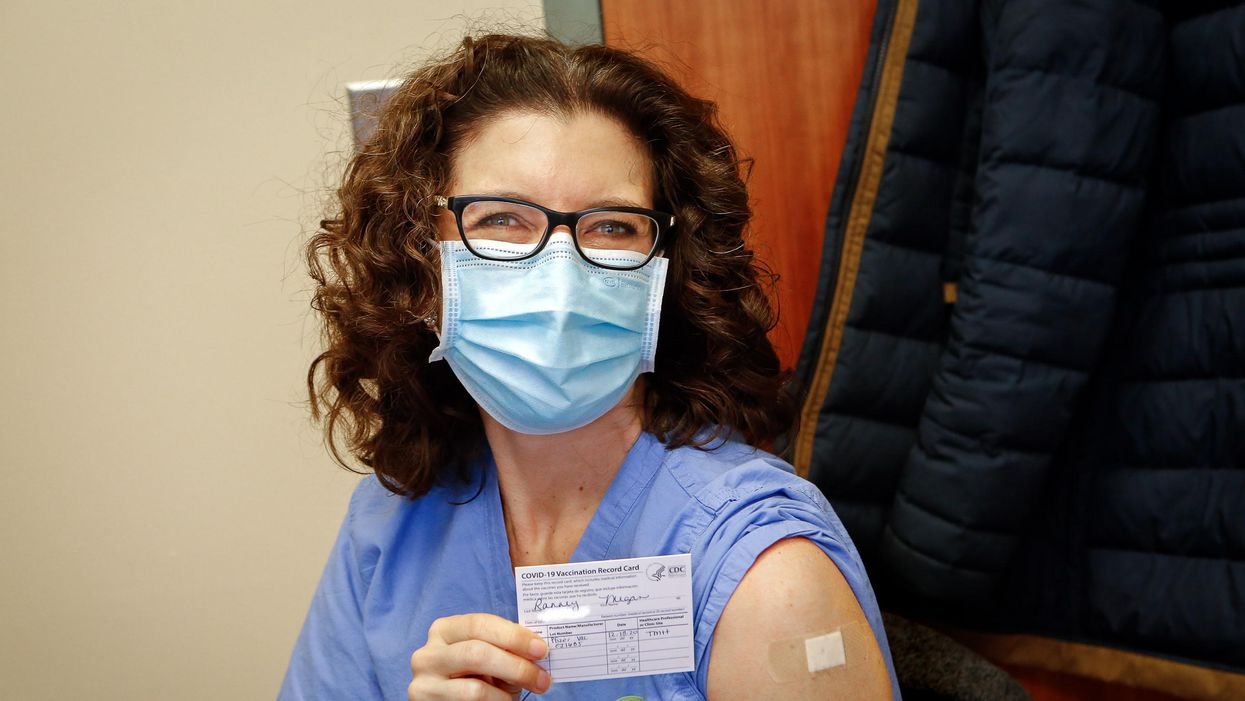
Dr. Ranney immediately after receiving her first dose of the Pfizer vaccine on December 18, 2020.
On December 18th, 2020, I received my first dose of the Pfizer mRNA vaccine against SARS-CoV-2. On January 9th, 2021, I received my second. I am now a CDC-card-carrying, fully vaccinated person.
The build-up to the first dose was momentous. I was scheduled for the first dose of the morning. Our vaccine clinic was abuzz with excitement and hope, and some media folks were there to capture the moment. A couple of fellow emergency physicians were in the same cohort of recipients as I; we exchanged virtual high-fives and took a picture of socially distanced hugs. It was, after all, the closest thing we'd had to a celebration in months.
I walked in the vaccine administration room with anticipation – it was tough to believe this moment was truly, finally here. I got a little video of my getting the shot, took my obligate vaccine selfie, waited in the observation area for 15 minutes to ensure I didn't have a reaction, and then proudly joined 1000s of fellow healthcare workers across the country in posting #ThisIsMyShot on social media. "Here we go, America!"
The first shot, though, didn't actually do all that much for me. It hurt less than a flu shot (which, by the way, doesn't hurt much). I had virtually no side effects. I also knew that it did not yet protect me. The Pfizer (and Moderna) data show very clearly that although the immune response starts to grow 10-12 days after the first shot, one doesn't reach full protection against COVID-19 until much later.
So when, two days after my first shot, I headed back to work in the emergency department, I kept wondering "Will this be the day that I get sick? Wouldn't that be ironic!" Although I never go without an N95 during patient care, it just takes one slip – scratching one's eyes, eating lunch in a break room that an infected colleague had just been in – to get ill. Ten months into this pandemic, it is so easy to get fatigued, to make a small error just one time.
Indeed, I had a few colleagues fall ill in between their first and second shots; one was hospitalized. This was not surprising, but still sad, given how close they had come to escaping infection.
Scientifically speaking, one doesn't need to feel bad to develop an immune response. Emotionally, though, I welcomed the symptoms as proof positive that I would be protected.
This time period felt a little like we had our learner's permit for driving: we were on our way to being safe, but not quite there yet.
I also watched, with dismay, our failures as a nation at timely distribution of the vaccine. On December 18th, despite the logistical snafus that many of us had started to highlight, it was still somewhat believable that we would at least distribute (if not actually administer) 20 million doses by the New Year. But by December 31, my worst fears about the feds' lack of planning had been realized. Only 14 million doses had gone to states, and fewer than 3 million had been administered. Within the public health and medical community, we began to debate how to handle the shortages and slow vaccination rates: should we change prioritization schemes? Get rid of the second dose, in contradiction to what our FDA had approved?
Let me be clear: I really, really, really wanted my second dose. It is what is supported by the data. After living this long at risk, it felt frankly unfair that I might not get fully protected. I waited with trepidation, afraid that policies would shift before I got it in my arm.
At last, my date for my second shot arrived.
This shot was a little less momentous on the outside. The vaccine clinic was much more crowded, as we were now administering first doses to more people, as well as providing the second dose to many. There were no high fives, no media, and I took no selfies. I finished my observation period without trouble (as did everyone else vaccinated the same day, as is typical for these vaccines). I walked out the door planning to spend a nice afternoon outdoors with my kids.
Within 15 minutes, though, the very common side effects – reported by 80% of people my age after the second dose – began to appear. First I got a headache (like 52% of people my age), then body aches (37%), fatigue (59%), and chills (35%). I felt "foggy", like I was fighting something. Like 45% of trial participants who had received the actual vaccine, I took acetaminophen and ibuprofen to stave off the symptoms. There is some minimal evidence from other vaccines that pre-treatment with these anti-inflammatories may reduce antibodies, but given that half of trial participants took these medications, there's no reason to make yourself suffer if you develop side effects. Forty-eight hours later, just in time for my next shift, the side effects magically cleared. Scientifically speaking, one doesn't need to feel bad to develop an immune response. Emotionally, though, I welcomed the symptoms as proof positive that I would be protected.
My reaction was truly typical. Although the media hype focuses on major negative reactions, they are – statistically speaking – tremendously rare: fewer than 11/million people who received the Pfizer vaccine, and 3/million who received the Moderna vaccine, developed anaphylaxis; of these, all were treated, and all are fine. Compare this with the fact that approximately 1200/million Americans have died of this virus. I'll choose the minor, temporary, utterly treatable side effects any day.
Now, more than 14 days after my second dose, the data says that my chance of getting really sick is, truly, infinitesimally low. I don't have to worry that each shift will put me into the hospital. I feel emotionally lighter, and a little bit like I have a secret super-power.
But I also know that we are not yet home free.
I may have my personal equivalent of Harry Potter's invisibility cloak – but we don't yet know whether it protects those around me, at all. As Dr. Fauci himself has written, while community spread is high, there is still a chance that I could be a carrier of infection to others. So I still wear my N95 at work, I still mask in public, and I still shower as soon as I get home from a shift and put my scrubs right in the washing machine to protect my husband and children. I also won't see my parents indoors until they, too, have been vaccinated.
At the end of the day, these vaccines are both amazing and life-changing, and not. My colleagues are getting sick less often, now that many of us are a week or more out from our second dose. I can do things (albeit still masked) that would simply not have been safe a month ago. These are small miracles, for which I am thankful. But like so many things in life, they would be better if shared with others. Only when my community is mostly vaccinated, will I breathe easy again.
My deepest hope is that we all have – and take - the chance to get our shots, soon. Because although the symbolism and effect of the vaccine is high, the experience itself was … not that big a deal.