The Nobel Prize-Winning Treatment Approach That Could Tackle COVID-19

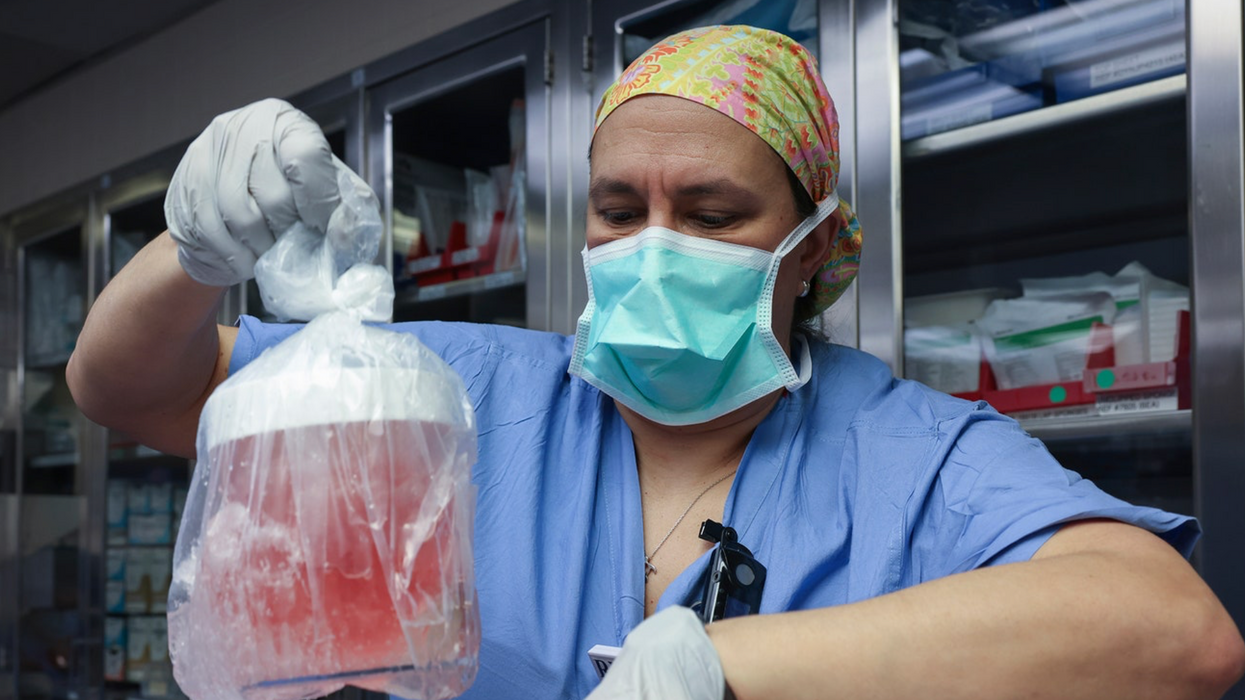
A researcher works in the lab at Alnylam Pharmaceuticals, which has pioneered the development of RNAi therapies.
In October 2006, Craig Mello received a strange phone call from Sweden at 4:30 a.m. The voice at the other end of the line told him to get dressed and that his life was about to change.
"We think this could be effective in [the early] phase, helping the body clear the virus and preventing progression to that severe hyperimmune response which occurs in some patients."
Shortly afterwards, he was informed that along with his colleague Andrew Fire, he had won the Nobel Prize in Physiology or Medicine.
Eight years earlier, biologists Fire and Mello had made a landmark discovery in the history of genetics. In a series of experiments conducted in worms, they had revealed an ancient evolutionary mechanism present in all animals that allows RNA – the structures within our cells that take genetic information from DNA and use it to make proteins – to selectively switch off genes.
At the time, scientists heralded the dawn of a new field of medical research utilizing this mechanism, known as RNA interference or RNAi, to tackle rare genetic diseases and deactivate viruses. Now, 14 years later, the pharmaceutical company Alnylam — which has pioneered the development of RNAi-based treatments over the past decade — is looking to use it to develop a groundbreaking drug for the virus that causes COVID-19.
"We can design small interfering RNAs to target regions of the viral genome and bind to them," said Akin Akinc, who manages several of Alnylam's drug development programs. "What we're learning about COVID-19 is that there's an early phase where there's lots of viral replication and a high viral load. We think this could be effective in that phase, helping the body clear the virus and preventing progression to that severe hyperimmune response which occurs in some patients."
Called ALN-COV, Alnylam's treatment hypothetically works by switching off a key gene in the virus, inhibiting its ability to replicate itself. In order to deliver it to the epithelial cells deep in the lung tissue, where the virus resides, patients will inhale a fine mist containing the RNAi molecules mixed in a saline solution, using a nebulizer.
But before human trials of the drug can begin, the company needs to convince regulators that it is both safe and effective in a series of preclinical trials. While early results appear promising - when mixed with the virus in a test tube, the drug displayed a 95 percent inhibition rate – experts are reserving judgment until it performs in clinical trials.
"If successful this could be a very important milestone in the development of RNAi therapies, but virus infections are very complicated and it can be hard to predict whether a given level of inhibition in cell culture will be sufficient to have a significant impact on the course of the infection," said Si-Ping Han, who researches RNAi therapeutics at California Institute of Technology and is not involved in the development of this drug.
So far, Alnylam has had success in using RNAi to treat rare genetic diseases. It currently has treatments licensed for Hereditary ATTR Amyloidosis and Acute Hepatic Porphyria. Another treatment, for Primary Hyperoxaluria Type 1, is currently under regulatory review. But its only previous attempt to use RNAi to tackle a respiratory infection was a failed effort to develop a drug for respiratory syncytial virus (RSV) almost a decade ago.
However, the technology has advanced considerably since then. "Back then, RNAi drugs had no chemical modifications whatsoever, so they were readily degraded by the body, and they could also result in unintended immune stimulation," said Akinc. "Since then, we've learned how to chemically modify our RNAi's to make them immunosilent and give them improved potency, stability, and duration of action."
"It would be a very important milestone in the development of RNAi therapies."
But one key challenge the company will face is the sheer speed at which viruses evolve, meaning they can become drug-resistant very quickly. Scientists predict that Alnylam will ultimately have to develop a series of RNAi drugs for the coronavirus that work together.
"There's been considerable interest in using RNAi to treat viral infections, as RNA therapies can be developed more rapidly than protein therapies like monoclonal antibodies, since one only needs to know the viral genome sequence to begin to design them," said David Schaffer, professor of bioengineering at University of California, Berkeley. "But viruses can evolve their sequences rapidly around single drugs so it is likely that a combinatorial RNAi therapy may be needed."
In the meantime, Alnylam is conducting further preclinical trials over the summer and fall, with the aim of launching testing in human volunteers by the end of this year -- an ambitious aim that would represent a breakneck pace for a drug development program.
If the approach does ultimately succeed, it would represent a major breakthrough for the field as a whole, potentially opening the door to a whole new wave of RNAi treatments for different lung infections and diseases.
"It would be a very important milestone in the development of RNAi therapies," said Han, the Caltech researcher. "It would be both the first time that an RNAi drug has been successfully used to treat a respiratory infection and as far as I know, the first time that one has been successful in treating any disease in the lungs. RNAi is a platform that can be reconfigured to hit different targets, and so once the first drug has been developed, we can expect a rapid flow of variants targeting other respiratory infections or other lung diseases."
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”
MILESTONE: Doctors have transplanted a pig organ into a human for the first time in history
A surgeon at Massachusetts General Hospital prepares a pig organ for transplant.
Surgeons at Massachusetts General Hospital made history last week when they successfully transplanted a pig kidney into a human patient for the first time ever.
The recipient was a 62-year-old man named Richard Slayman who had been living with end-stage kidney disease caused by diabetes. While Slayman had received a kidney transplant in 2018 from a human donor, his diabetes ultimately caused the kidney to fail less than five years after the transplant. Slayman had undergone dialysis ever since—a procedure that uses an artificial kidney to remove waste products from a person’s blood when the kidneys are unable to—but the dialysis frequently caused blood clots and other complications that landed him in the hospital multiple times.
As a last resort, Slayman’s kidney specialist suggested a transplant using a pig kidney provided by eGenesis, a pharmaceutical company based in Cambridge, Mass. The highly experimental surgery was made possible with the Food and Drug Administration’s “compassionate use” initiative, which allows patients with life-threatening medical conditions access to experimental treatments.
The new frontier of organ donation
Like Slayman, more than 100,000 people are currently on the national organ transplant waiting list, and roughly 17 people die every day waiting for an available organ. To make up for the shortage of human organs, scientists have been experimenting for the past several decades with using organs from animals such as pigs—a new field of medicine known as xenotransplantation. But putting an animal organ into a human body is much more complicated than it might appear, experts say.
“The human immune system reacts incredibly violently to a pig organ, much more so than a human organ,” said Dr. Joren Madsen, director of the Mass General Transplant Center. Even with immunosuppressant drugs that suppress the body’s ability to reject the transplant organ, Madsen said, a human body would reject an animal organ “within minutes.”
So scientists have had to use gene-editing technology to change the animal organs so that they would work inside a human body. The pig kidney in Slayman’s surgery, for instance, had been genetically altered using CRISPR-Cas9 technology to remove harmful pig genes and add human ones. The kidney was also edited to remove pig viruses that could potentially infect a human after transplant.
With CRISPR technology, scientists have been able to prove that interspecies organ transplants are not only possible, but may be able to successfully work long term, too. In the past several years, scientists were able to transplant a pig kidney into a monkey and have the monkey survive for more than two years. More recently, doctors have transplanted pig hearts into human beings—though each recipient of a pig heart only managed to live a couple of months after the transplant. In one of the patients, researchers noted evidence of a pig virus in the man’s heart that had not been identified before the surgery and could be a possible explanation for his heart failure.
So far, so good
Slayman and his medical team ultimately decided to pursue the surgery—and the risk paid off. When the pig organ started producing urine at the end of the four-hour surgery, the entire operating room erupted in applause.
Slayman is currently receiving an infusion of immunosuppressant drugs to prevent the kidney from being rejected, while his doctors monitor the kidney’s function with frequent ultrasounds. Slayman is reported to be “recovering well” at Massachusetts General Hospital and is expected to be discharged within the next several days.