The Sickest Babies Are Covered in Wires. New Tech Is Changing That.
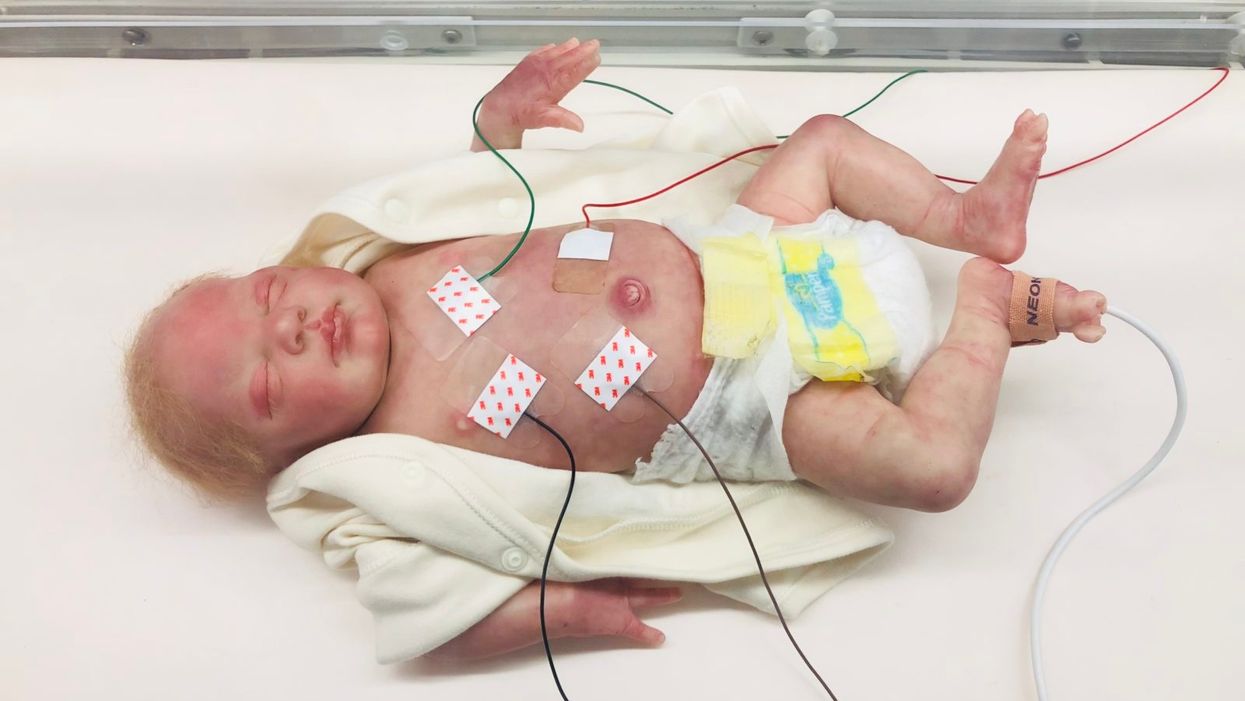
A wired baby in a neonatal intensive care unit.
I'll never forget the experience of having a child in the neonatal intensive care unit (NICU).
Now more than ever, we're working to remove the barriers between new parents and their infants.
It was another layer of uncertainty that filtered into my experience of being a first-time parent. There was so much I didn't know, and the wires attached to my son's small body for the first week of his life were a reminder of that.
I wanted to be the best mother possible. I deeply desired to bring my son home to start our lives. More than anything, I longed for a wireless baby whom I could hold and love freely without limitations.
The wires suggested my baby was fragile and it left me feeling severely unprepared, anxious, and depressed.
In recent years, research has documented the ways that NICU experiences take a toll on parents' mental health. But thankfully, medical technology is rapidly being developed to help reduce the emotional fallout of the NICU. Now more than ever, we're working to remove the barriers between new parents and their infants. The latest example is the first ever wireless monitoring system that was recently developed by a team at Northwestern University.
After listening to the needs of parents and medical staff, Debra Weese-Mayer, M.D., a professor of pediatric autonomic medicine at Feinberg School of Medicine, along with a team of materials scientists, engineers, dermatologists and pediatricians, set out to develop this potentially life-changing technology. Weese-Mayer believes wireless monitoring will have a significant impact for people on all sides of the NICU experience.
"With elimination of the cumbersome wires," she says, "the parents will find their infant more approachable/less intimidating and have improved access to their long-awaited but delivered-too-early infant, allowing them to begin skin-to-skin contact and holding with reduced concern for dislodging wires."
So how does the new system work?
Very thin "skin like" patches made of silicon rubber are placed on the surface of the skin to monitor vitals like heart rate, respiration rate, and body temperature. One patch is placed on the chest or back and the other is placed on the foot.
These patches are safer on the skin than previously used adhesives, reducing the cuts and infections associated with past methods. Finally, an antenna continuously delivers power, often from under the mattress.
The data collected from the patches stream from the body to a tablet or computer.

New wireless sensor technology is being studied to replace wired monitoring in NICUs in the coming years.
(Northwestern University)
Weese-Mayer hopes that wireless systems will be standard soon, but first they must undergo more thorough testing. "I would hope that in the next five years, wireless monitoring will be the standard in NICUs, but there are many essential validation steps before this technology will be embraced nationally," she says.
Until the new systems are ready, parents will be left struggling with the obstacles that wired monitoring presents.
Physical intimacy, for example, appears to have pain-reducing qualities -- something that is particularly important for babies who are battling serious illness. But wires make those cuddles more challenging.
There's also been minimal discussion about how wired monitoring can be particularly limiting for parents with disabilities and mobility aids, or even C-sections.
"When he was first born and I was recovering from my c-section, I couldn't deal with keeping the wires untangled while trying to sit down without hurting myself," says Rhiannon Giles, a writer from North Carolina, who delivered her son at just over 31 weeks after suffering from severe preeclampsia.
"The wires were awful," she remembers. "They fell off constantly when I shifted positions or he kicked a leg, which meant the monitors would alarm. It felt like an intrusion into the quiet little world I was trying to mentally create for us."
Over the last few years, researchers have begun to dive deeper into the literal and metaphorical challenges of wired monitoring.
For many parents, the wires prompt anxiety that worsens an already tense and vulnerable time.
I'll never forget the first time I got to hold my son without wires. It was the first time that motherhood felt manageable.
"Seeing my five-pound-babies covered in wires from head to toe rendered me completely overwhelmed," recalls Caila Smith, a mom of five from Indiana, whose NICU experience began when her twins were born pre-term. "The nurses seemed to handle them perfectly, but I was scared to touch them while they appeared so medically frail."
During the nine days it took for both twins to come home, the limited access she had to her babies started to impact her mental health. "If we would've had wireless sensors and monitors, it would've given us a much greater sense of freedom and confidence when snuggling our newborns," Smith says.
Besides enabling more natural interactions, wireless monitoring would make basic caregiving tasks much easier, like putting on a onesie.
"One thing I noticed is that many preemie outfits are made with zippers," points out Giles, "which just don't work well when your baby has wires coming off of them, head to toe."
Wired systems can pose issues for medical staff as well as parents.
"The main concern regarding wired systems is that they restrict access to the baby and often get tangled with other equipment, like IV lines," says Lamia Soghier, Medical Director of the Neonatal Intensive Care Unit at Children's National in Washington, D.C , who was also a NICU parent herself. "The nurses have to untangle the wires, which takes time, before handing the baby to the family."
I'll never forget the first time I got to hold my son without wires. It was the first time that motherhood felt manageable, and I couldn't stop myself from crying. Suddenly, anything felt possible and all the limitations from that first week of life seemed to fade away. The rise of wired-free monitoring will make some of the stressors that accompany NICU stays a thing of the past.
Move Over, Iron Man. A Real-Life Power Suit Helped This Paralyzed Grandmother Learn to Run.
Puschel Sorensen undergoing physical therapy using Cyberdyne's hybrid assistive limb, left, and with her grandson while still in a wheelchair.
Puschel Sorensen first noticed something was wrong when her fingertips began to tingle. Later that day, she grew weak and fell.
It picked up small electrical impulses on her skin's surface and turned them into full movement in her legs.
Her family rushed her to the doctor, where she received the devastating diagnosis of Guillain-Barré Syndrome -- a rare and rapidly progressing autoimmune disorder that attacks the myelin sheath covering nerves.
Sorensen, a once-spry grandmother in her late fifties, spent 54 days in intensive care in 2018. When she was finally transferred to a rehab facility near her home in Florida, she was still on a feeding tube and ventilator, and was paralyzed from the neck down. Progress with traditional physical therapy was slow.

Sorensen in the hospital after her diagnosis of Guillain-Barré syndrome.
And then everything changed. Sorensen began using a cutting-edge technology called an exoskeleton to relearn how to walk. In the vein of Iron Man's fictional power suit, it confers strength and mobility to the wearer that isn't possible otherwise. In Sorensen's case, her device, called HAL – for hybrid assistive limb -- picked up small electrical impulses on her skin's surface and turned them into full movement in her legs while she attempted to walk on a treadmill.
"It was very difficult, but super awesome," recalls Sorensen, of first using the device. "The robot was having to do all the work for me."
Amazingly, within a year, she was running. She's one of 38 patients who have used HAL to recover from accidents or medical catastrophes.
"How do you thank someone for giving them back the ability to walk, the ability to live your life again?" Sorensen asks effusively.
It's still early days for such exoskeleton devices, which number perhaps a few thousand worldwide, according to data from the handful of manufacturers who create them with any scale. But the devices' ability to dramatically rehabilitate patients like Sorensen highlights their potential to extract untold numbers of people from wheelchairs, and even to usher in a new paradigm for caregiving – one of the fastest growing segments of the U.S. economy.
"I've been a physical therapist for 16 years, and (these devices) help teach patients the right way to move in rehabilitation," says Robert McIver, director of clinical technology at the Brooks Cybernic Treatment Center, part of the Brooks Rehabilitation Hospital in Jacksonville, Fla, where Sorensen recovered.
Another patient there, a 17-year-old named George with a snowboarding injury that paralyzed his legs, was getting around with a walker within 20 sessions.
As patients progress in their recoveries, so does exoskeleton technology. Jack Peurach, CEO of Ekso, one of the leaders in the space, believes within a decade they could resemble an article of clothing (a "magic pair of pants" is his phrase). They also may become inexpensive and reliable enough to transition from a medical to a consumer device. McIver sees them eventually being used in the home on an ongoing basis as a personal assistive device, much like a walker or cane, to prevent falls in elderly people.
Such a transition "certainly could eventually lessen the need for caregivers," says Sharona Hoffman, a professor of law at Case Western University in Cleveland who has written extensively on aging and bioethics. "We have a real shortage of caregivers, so that would be a good thing."
Of course, having an aging and disabled population using exoskeletons in much the same way as an Apple Watch raises issues of its own.
Dr. Elizabeth Landsverk, a California-based geriatrician and founder of a company that performs house calls for elderly patients, believes the tech holds some promise in easing the burden on caregivers, who sometimes have to lift or move patients without assistance. But she also believes exoskeletons could become overhyped.
"I don't see robotics as completely replacing the caregiver," she says. And even if exoskeletons became akin to articles of clothing, she is skeptical of how convenient they could become.
"It's hard enough to get into support hose. Would an older person be able to get in and out of it on their own?" she asks, noting that a patient's cognitive levels could pose a huge barrier to donning such a device without assistance.
If personal exoskeletons did wildly succeed, Hoffman wonders whether they would leave the elderly more physically mobile yet also more socially isolated, since caregivers or even residing in an assisted living facility may no longer be required. Or, if they were priced in the hundreds or thousands of dollars, he worries that the cost would exacerbate social inequalities among the elderly and disabled.
"It's almost like a bad dream that [my illness] happened."
With any technology that confers superhuman ability, there's also the question of appropriate usage. Even the fictional Power Loader in the movie Alien required an operator's license. In the real world, such an approach would likely pay dividends.
"We would have to make sure physicians are well-trained in these devices, and patients have a way of getting training to operate them that is thorough and responsible," Hoffman says.
But despite some unresolved questions, it is a remarkable achievement to be able to give people back their lives thanks to new technology.
"It's almost like a bad dream that [my illness] happened," says Sorensen, who managed to walk in her daughter's wedding after her recovery. "Because now everything is pretty much back to normal and it's awesome."
23andMe Is Using Customers’ Genetic Data to Develop Drugs. Is This Brilliant or Dubious?
A woman does a DNA test with a cotton swab at home.
Leading direct-to-consumer (DTC) genetic testing companies are continuously unveiling novel ways to leverage their vast stores of genetic data.
"23andMe will tell you what diseases you have and then sell you the drugs to treat them."
As reported last week, 23andMe's latest concept is to develop and license new drugs using the data of consumers who have opted in to let their information be used for research. To date, over 10 million people have used the service and around 80 percent have opted in, making its database one of the largest in the world.
Culture researcher Dr. Julia Creet is one of the foremost experts on the DTC genetic testing industry, and in her forthcoming book, The Genealogical Sublime, she bluntly examines whether such companies' motives and interests are in sync with those of consumers.
Leapsmag caught up with Creet about the latest news and the wider industry's implications for health and privacy.
23andMe has just announced that it plans to license a newly developed anti-inflammatory drug, the first one created using its customers' genetic data, to Almirall, a pharma company in Spain. What's your take?
I think this development is the next step in the evolution of the company and its "double-sided" marketing model. In the past, as it enticed customers to give it their DNA, it sold the results and the medical information divulged by customers to other drug companies. Now it is positioning itself to reap the profits of a new model by developing treatments itself.
Given that there are many anti-inflammatory drugs on the market already, whatever Almirall produces might not have much of an impact. We might see this canny move as a "proof of concept," that 23andMe has learned how to "leverage" its genetic data without having to sell them to a third party. In a way, the privacy provisions will be much less complicated, and the company stands to attract investment as it turns itself into [a pseudo pharmaceutical company], a "pharma-psuedocal" company.
Emily Drabant Conley, the president of business development, has said that 23andMe is pursuing other drug compounds and may conduct their own clinical trials rather than licensing them out to their existing research partners. The end goal, it seems, is to make direct-to-consumer DNA testing to drug production and sales back to that same consumer base a seamless and lucrative circle. You have to admit it's a brilliant business model. 23andMe will tell you what diseases you have and then sell you the drugs to treat them.
In your new book, you describe how DTC genetic testing companies have capitalized on our innate human desire to connect with or ancestors and each other. I quote you: "This industry has taken that potent, spiritual, all-too-human need to belong... and monetized it in a particularly exploitative way." But others argue that DTC genetic testing companies are merely providing a service in exchange for fair-market compensation. So where does exploitation come into the picture?
Yes, the industry provides a fee for service, but that's only part of the story. The rest of the story reveals a pernicious industry that hides its business model behind the larger science project of health and heredity. All of the major testing companies play on the idea of "lack," that we can't know who we are unless we buy information about ourselves. When you really think about it, "Who do you think you are?" is a pernicious question that suggests that we don't or can't know who we or to whom we are related without advanced data searches and testing. This existential question used to be a philosophical question; now the answers are provided by databases that acquire more valuable information than they provide in the exchange.
"It's a brilliant business model that exploits consumer naiveté."
As you've said before, consumers are actually paying to be the product because the companies are likely to profit more from selling their genetic data. Could you elaborate?
The largest databases, AncestryDNA and 23andMe, have signed lucrative agreements with biotech companies that pay them for the de-identified data of their customers. What's so valuable is the DNA combined with the family relationships. Consumers provide the family relationships and the companies link and extrapolate the results to larger and larger family trees. Combined with the genetic markers for certain diseases, or increased susceptibility to certain diseases, these databases are very valuable for biotech research.
None of that value will ever be returned to consumers except in the form of for-profit drugs. Ancestry, in particular, has removed all information about its "research partners" from its website, making it very difficult to see how it is profiting from its third-party sales. 23andMe is more open about its "two-sided business model," but encourages consumers to donate their information to science. It's a brilliant business model that exploits consumer naiveté.
A WIRED journalist wrote that "23andMe has been sharing insights gleaned from consented customer data with GSK and at least six other pharmaceutical and biotechnology firms for the past three and a half years." Is this a consumer privacy risk?
I don't see that 23andMe did anything to which consumers didn't consent, albeit through arguably unreadable terms and conditions. The part that worries me more is the 300 phenotype data points that the company has collected on its consumers through longitudinal surveys designed, as Anne Wojcicki, CEO and Co-founder of 23andMe, put it, "to circumvent medical records and just self-report."
Everyone is focused on the DNA, but it's the combination of genetic samples, genealogical information and health records that is the most potent dataset, and 23andMe has figured out a way to extract all three from consumers.


