Virtual Reality is Making Medical Care for Kids Less Scary and Painful
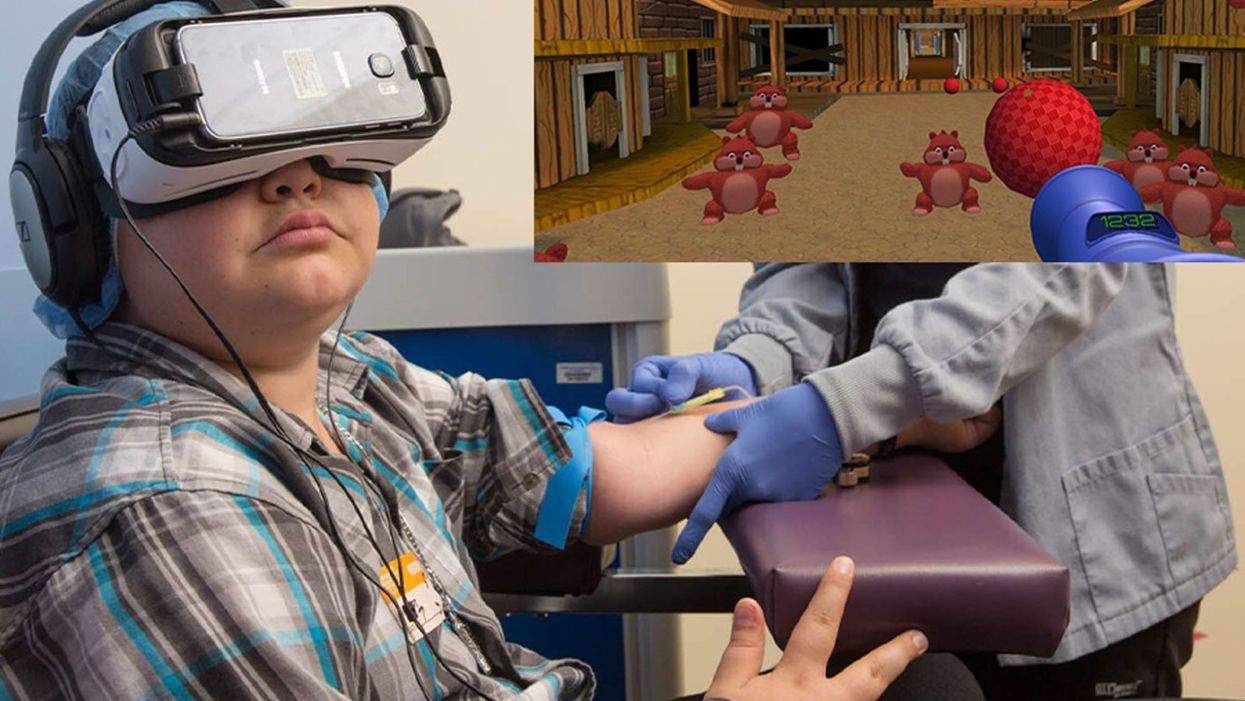
A patient at Children's Hospital Los Angeles (Courtesy of Children's Hospital Los Angeles) plus Bear Blast, developed by AppliedVR.
A blood draw is not normally a fun experience, but these days, virtual reality technology is changing that.
Instead of watching a needle go into his arm, a child wearing a VR headset at Children's Hospital Los Angeles can play a game throwing balls at cartoon bears. In Seattle, at the University of Washington, a burn patient can immerse herself in a soothing snow scene. And at the University of Miami Hospital, a five-minute skin biopsy can become an exciting ride at an amusement park.
VR is transforming once-frightening medical encounters for kids, from blood draws to biopsies to pre-surgical prep, into tolerable ones.
It's literally a game changer, says pediatric neurosurgeon Kurtis Auguste, who uses the tool to help explain pending operations to his young patients and their families. The virtual reality 3-D portrait of their brain is recreated from an MRI, originally to help plan the surgery. The image of normally bland tissue is painted with false colors to better see the boundaries and anomalies of each component. It can be rotated, viewed from every possible angle, zoomed in and out; incisions can be made and likely results anticipated. Auguste has extended its use to patients and families.
"The moment you put these headsets on the kids, we immediately have a link, because honestly, this is how they communicate with each other," says Auguste. "We're all sitting around the table playing games. It's really bridged the distance between me, the pediatric specialist, and my patients" at the Benioff Children's Hospital Oakland, now affiliated with the University of California San Francisco School of Medicine.
The VR experience engages people where they are, immersing them in the environment rather than lecturing them. And it seems to work in all environments, across age and cultural differences, leading to a better grasp of what will be undertaken. That understanding is crucial to meaningful informed consent for surgery. It is particularly relevant for safety-net hospitals, which includes most children's hospitals, because often members of the families were born elsewhere and may have limited understanding of English, not to mention advanced medicine.
Targeting pain
"We're trying to target ways that we can decrease pain, anxiety, fear – what people usually experience as a function of a needle," says Jeffrey Gold, a pioneer in adapting VR at Children's Hospital Los Angeles. He ran the pain clinic there and in 2004 initially focused on phlebotomy, simple blood draws. Many of their kids require frequent blood draws to monitor serious chronic conditions such as diabetes, HIV infection, sickle cell disease, and other conditions that affect the heart, liver, kidneys and other organs.
The scientific explanation of how VR works for pain relief draws upon two basic principles of brain function. The first is "top down inhibition," Gold explains. "We all have the inherent capacity to turn down signals once we determine that signal is no longer harmful, dangerous, hurtful, etc. That's how our brain operates on purpose. It's not just a distraction, it's actually your brain stopping the pain signal at the spinal cord before it can fire all the way up to the frontal lobe."
Second is the analgesic effect from endorphins. "If you're in a gaming environment, and you're having fun and you're laughing and giggling, you are actually releasing endorphins...a neurochemical reaction at the synaptic level of the brain," he says.
Part of what makes VR effective is "what's called a cognitive load, where you have to actually learn something and do something," says Gold. He has worked with developers on a game call Bear Blast, which has proven to be effective in a clinical trial for mitigating pain. But he emphasizes, it is not a one-size-fits all; the programs and patients need to be evaluated to understand what works best for each case.
Gold was a bit surprised to find that VR "actually facilitates quicker blood draws," because the staff doesn't have to manage the kids' anxiety, so "they require fewer needle sticks." The kids, parents, and staff were all having a good time, "and that's a big win when everybody is benefiting." About two years ago the hospital made VR an option that patients can request in the phlebotomy lab, and about half of kids age 4 and older choose to do so.
The technology "gets the kids engaged and performing the activity the way we want them to" to maximize recovery.
VR reduces or eliminates the need to use sedation or anesthesia, which carries a small but real risk of an adverse reaction. And important to parents, it eliminates the recovery time from using sedation, which shortens the visit and time missed from school and work.
A more intriguing question is whether reducing fear and anxiety in early-life experiences with the healthcare system through activities like VR will have a long-term affect on kids' attitudes toward medicine as they grow older. "If you're a screaming meemie when you come get your blood draw when you're five or seven, you're still that anxious adolescent or adult who is all quivering and sweating and avoiding healthcare," Gold says. "That's a longitudinal health outcome I'd love to get my hands on in 10-15 years from now."
Broader applications
Dermatologist Hadar Lev-Tov read about the use of VR to treat pain and decided to try it in his practice at the University of Miami Hospital. He thought, "OK, this is low risk, it's easy to do. So we got some equipment and got it done." It was so affordable he paid for it out of his own pocket, rather than wait to go through administrative channels. The results were so interesting that he decided to publish it as a series of case studies with a wide variety of patients and types of procedures.
Some of them, such as freezing off warts, are not particularly painful. "But there can be a lot of anxiety, especially for kids, which can be worse than pain and can disrupt the procedure." It can trigger a non-rational, primal fight or flight response in the limbic region of the brain.
Adults understand the need for a biopsy of a skin growth and tolerate what might be a momentary flick of pain. "But for a kid you think twice about a biopsy, both because it's a hassle and because it could be a traumatic event for a child," says Lev-Tov. VR has helped to allay such fears and improve medical care.
Integrating VR into practice has been relatively easy, primarily focusing on simple training for staff and ensuring that standard infection control practices are used in handling equipment that is used by different patients. More mundane issues are ensuring that the play back and wi-fi equipment are functioning properly. He has had a few complaints from kids when the procedure is competed and the VR is turned off prematurely, which is why he favors programs like a roller coaster ride that lasts about five minutes, ample time to take a biopsy or two.
The future is today
The pediatric neurosurgeon Auguste is collaborating with colleagues at Oakland Children's to expand use of VR into different areas of care. Cancer specialists often use a port, a bubble installed under the skin in the chest of the child, to administer chemotherapy. But the young patient's curiosity often draws their attention downward to the port and their chin can potentially contaminate or obstruct it, interfering with the procedure. So the team developed a VR game involving birds that requires players to move their heads upward, away from the port, improving administration of the drugs and reducing the risk of infection.
Innovative use of VR just may be one tool that actually makes kids eager to visit the doctor.
Other games are being developed for rehabilitation that require the use of specific nerve and muscle combinations. The technology "gets the kids engaged and performing the activity the way we want them to" to maximize recovery, Auguste explains. "We can monitor their progress by the score on the game, and if it plateaus, maybe switch to another game."
Another project is trying to ease the anxiety and confusion of the patient and family experience within the hospital itself. Hospital staff are creating a personalized VR introductory walking tour that leads from the parking garage through the maze of structures and corridors in the hospital complex to Dr. Auguste's office, phlebotomy, the MRI site, and other locations they might visit. The goal is to make them familiar with key landmarks before they even set foot in the facility. "So when they come the day of the visit they have already taken that exact same path, hopefully more than once."
"They don't miss their MRI appointment and therefore they don't miss their clinical appointment with me," says Auguste. It reduces patient anxiety about the encounter and from the hospital's perspective, it will reduce costs of missed and rescheduled visits simply because patients did not go to the right place at the right time.
The VR visit will be emailed to patients ahead of time and they can watch it on a smartphone installed in a disposable cardboard viewer. Oakland Children's hopes to have the system in place by early next year. Auguste says their goal in using VR, like other health care providers across the country, is "to streamline the entire patient experience."
Innovative use of VR just may be one tool that actually makes kids eager to visit the doctor. That would be a boon to kids, parents, and the health of America.
In this week's Friday Five, new research that could help prevent Alzheimer's. Plus, why you should care about smart senior towns, how to reverse being drunk, money can make you happier if you're this type of person, and personalized anxiety medicine.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Here are the promising studies covered in this week's Friday Five, featuring interviews with Dr. Christopher Martens, director of the Delaware Center for Cogntiive Aging Research and professor of kinesiology and applied physiology at the University of Delaware, and Dr. Ilona Matysiak, visiting scholar at Iowa State University and associate professor of sociology at Maria Grzegorzewska University.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
- Could this supplement help prevent Alzheimer's?
- Why you should care about smart senior towns
- Here's how to reverse being drunk
- Money can make you happy - if you're this type of person
- Personalized anxiety medicine
As gene therapies and small molecule drugs are being studied in clinical trials, companies increasingly see the value in hiring patients to help explain the potential benefits.
As a child, Wendy Borsari participated in a health study at Boston Children’s Hospital. She was involved because heart disease and sudden cardiac arrest ran in her family as far back as seven generations. When she was 18, however, the study’s doctors told her that she had a perfectly healthy heart and didn’t have to worry.
A couple of years after graduating from college, though, the Boston native began to experience episodes of near fainting. During any sort of strenuous exercise, my blood pressure would drop instead of increasing, she recalls.
She was diagnosed at 24 with hypertrophic cardiomyopathy. Although HCM is a commonly inherited heart disease, Borsari’s case resulted from a rare gene mutation, the MYH7 gene. Her mother had been diagnosed at 27, and Borsari had already lost her grandmother and two maternal uncles to the condition. After her own diagnosis, Borsari spent most of her free time researching the disease and “figuring out how to have this condition and still be the person I wanted to be,” she says.
Then, her son was found to have the genetic mutation at birth and diagnosed with HCM at 15. Her daughter, also diagnosed at birth, later suffered five cardiac arrests.
That changed Borsari’s perspective. She decided to become a patient advocate. “I didn’t want to just be a patient with the condition,” she says. “I wanted to be more involved with the science and the biopharmaceutical industry so I could be active in helping to make it better for other patients.”
She consulted on patient advocacy for a pharmaceutical and two foundations before coming to a company called Tenaya in 2021.
“One of our core values as a company is putting patients first,” says Tenaya's CEO, Faraz Ali. “We thought of no better way to put our money where our mouth is than by bringing in somebody who is affected and whose family is affected by a genetic form of cardiomyopathy to have them make sure we’re incorporating the voice of the patient.”
Biomedical corporations and government research agencies are now incorporating patient advocacy more than ever, says Alice Lara, president and CEO of the Sudden Arrhythmia Death Syndromes Foundation in Salt Lake City, Utah. These organizations have seen the effectiveness of including patient voices to communicate and exemplify the benefits that key academic research institutions have shown in their medical studies.
“From our side of the aisle,” Lara says, “what we know as patient advocacy organizations is that educated patients do a lot better. They have a better course in their therapy and their condition, and understanding the genetics is important because all of our conditions are genetic.”
Founded in 2016, Tenaya is advancing gene therapies and small molecule drugs in clinical trials for both prevalent and rare forms of heart disease, says Ali, the CEO.
The firm's first small molecule, now in a Phase 1 clinical trial, is intended to treat heart failure with preserved ejection fraction, where the amount of blood pumped by the heart is reduced due to the heart chambers becoming weak or stiff. The condition accounts for half or more of all heart failure in the U.S., according to Ali, and is growing quickly because it's closely associated with diabetes. It’s also linked with metabolic syndrome, or a cluster of conditions including high blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol levels.
“We have a novel molecule that is first in class and, to our knowledge, best in class to tackle that, so we’re very excited about the clinical trial,” Ali says.
The first phase of the trial is being performed with healthy participants, rather than people with the disease, to establish safety and tolerability. The researchers can also look for the drug in blood samples, which could tell them whether it's reaching its target. Ali estimates that, if the company can establish safety and that it engages the right parts of the body, it will likely begin dosing patients with the disease in 2024.
Tenaya’s therapy delivers a healthy copy of the gene so that it makes a copy of the protein missing from the patients' hearts because of their mutation. The study will start with adult patients, then pivot potentially to children and even newborns, Ali says, “where there is an even greater unmet need because the disease progresses so fast that they have no options.”
Although this work still has a long way to go, Ali is excited about the potential because the gene therapy achieved positive results in the preclinical mouse trial. This animal trial demonstrated that the treatment reduced enlarged hearts, reversed electrophysiological abnormalities, and improved the functioning of the heart by increasing the ejection fraction after the single-dose of gene therapy. That measurement remained stable to the end of the animals’ lives, roughly 18 months, Ali says.
He’s also energized by the fact that heart disease has “taken a page out of the oncology playbook” by leveraging genetic research to develop more precise and targeted drugs and gene therapies.
“Now we are talking about a potential cure of a disease for which there was no cure and using a very novel concept,” says Melind Desai of the Cleveland Clinic.
Tenaya’s second program focuses on developing a gene therapy to mitigate the leading cause of hypertrophic cardiomyopathy through a specific gene called MYPBC3. The disease affects approximately 600,000 patients in the U.S. This particular genetic form, Ali explains, affects about 115,000 in the U.S. alone, so it is considered a rare disease.
“There are infants who are dying within the first weeks to months of life as a result of this mutation,” he says. “There are also adults who start having symptoms in their 20s, 30s and 40s with early morbidity and mortality.” Tenaya plans to apply before the end of this year to get the FDA’s approval to administer an investigational drug for this disease humans. If approved, the company will begin to dose patients in 2023.
“We now understand the genetics of the heart much better,” he says. “We now understand the leading genetic causes of hypertrophic myopathy, dilated cardiomyopathy and others, so that gives us the ability to take these large populations and stratify them rationally into subpopulations.”
Melind Desai, MD, who directs Cleveland Clinic’s Hypertrophic Cardiomyopathy Center, says that the goal of Tenaya’s second clinical study is to help improve the basic cardiac structure in patients with hypertrophic cardiomyopathy related to the MYPBC3 mutation.
“Now we are talking about a potential cure of a disease for which there was no cure and using a very novel concept,” he says. “So this is an exciting new frontier of therapeutic investigation for MYPBC3 gene-positive patients with a chance for a cure.
Neither of Tenaya’s two therapies address the gene mutation that has affected Borsari and her family. But Ali sees opportunity down the road to develop a gene therapy for her particular gene mutation, since it is the second leading cause of cardiomyopathy. Treating the MYH7 gene is especially challenging because it requires gene editing or silencing, instead of just replacing the gene.

Wendy Borsari was diagnosed at age 24 with a commonly inherited heart disease. She joined Tenaya as a patient advocate in 2021.
Wendy Borsari
“If you add a healthy gene it will produce healthy copies,” Ali explains, “but it won’t stop the bad effects of the mutant protein the gene produces. You can only do that by silencing the gene or editing it out, which is a different, more complicated approach.”
Euan Ashley, professor of medicine and genetics at Stanford University and founding director of its Center for Inherited Cardiovascular Disease, is confident that we will see genetic therapies for heart disease within the next decade.
“We are at this really exciting moment in time where we have diseases that have been under-recognized and undervalued now being attacked by multiple companies with really modern tools,” says Ashley, author of The Genome Odyssey. “Gene therapies are unusual in the sense that they can reverse the cause of the disease, so we have the enticing possibility of actually reversing or maybe even curing these diseases.”
Although no one is doing extensive research into a gene therapy for her particular mutation yet, Borsari remains hopeful, knowing that companies such as Tenaya are moving in that direction.
“I know that’s now on the horizon,” she says. “It’s not just some pipe dream, but will happen hopefully in my lifetime or my kids’ lifetime to help them.”

