Will Blockchain Technology Usher in a Healthcare Data Revolution?

Digital blockchain concept.
The hacker collective known as the Dark Overlord first surfaced in June 2016, when it advertised more than 600,000 patient files from three U.S. healthcare organizations for sale on the dark web. The group, which also attempted to extort ransom from its victims, soon offered another 9 million records pilfered from health insurance companies and provider networks across the country.
Since 2009, federal regulators have counted nearly 5,000 major data breaches in the United States alone, affecting some 260 million individuals.
Last October, apparently seeking publicity as well as cash, the hackers stole a trove of potentially scandalous data from a celebrity plastic surgery clinic in London—including photos of in-progress genitalia- and breast-enhancement surgeries. "We have TBs [terabytes] of this shit. Databases, names, everything," a gang representative told a reporter. "There are some royal families in here."
Bandits like these are prowling healthcare's digital highways in growing numbers. Since 2009, federal regulators have counted nearly 5,000 major data breaches in the United States alone, affecting some 260 million individuals. Although hacker incidents represent less than 20 percent of the total breaches, they account for almost 80 percent of the affected patients. Such attacks expose patients to potential blackmail or identity theft, enable criminals to commit medical fraud or file false tax returns, and may even allow hostile state actors to sabotage electric grids or other infrastructure by e-mailing employees malware disguised as medical notices. According to the consulting agency Accenture, data theft will cost the healthcare industry $305 billion between 2015 and 2019, with annual totals doubling from $40 billion to $80 billion.
Blockchain could put patients in control of their own data, empowering them to access, share, and even sell their medical information as they see fit.
One possible solution to this crisis involves radically retooling the way healthcare data is stored and shared—by using blockchain, the still-emerging information technology that underlies cryptocurrencies such as Bitcoin. And blockchain-enabled IT systems, boosters say, could do much more than prevent the theft of medical data. Such networks could revolutionize healthcare delivery on many levels, creating efficiencies that would reduce medical errors, improve coordination between providers, drive down costs, and give researchers unprecedented insights into patterns of disease. Perhaps most transformative, blockchain could put patients in control of their own data, empowering them to access, share, and even sell their medical information as they see fit. Widespread adoption could result in "a new kind of healthcare economy, in which data and services are quantifiable and exchangeable, with strong guarantees around both the security and privacy of sensitive information," wrote W. Brian Smith, chief scientist of healthcare-blockchain startup PokitDok, in a recent white paper.
Around the world, entrepreneurs, corporations, and government agencies are hopping aboard the blockchain train. A survey by the IBM Institute for Business Value, released in late 2016, found that 16 percent of healthcare executives in 16 countries planned to begin implementing some form of the technology in the coming year; 90 percent planned to launch a pilot program in the next two years. In 2017, Estonia became the first country to switch its medical-records system to a blockchain-based framework. Great Britain and Dubai are exploring a similar move. Yet in countries with more fragmented health systems, most notably the U.S., the challenges remain formidable. Some of the most advanced healthcare applications envisioned for blockchain, moreover, raise technological and ethical questions whose answers may not arrive anytime soon.
By creating a detailed, comprehensive, and immutable timeline of medical transactions, blockchain-based recordkeeping could help providers gauge a patient's long-term health patterns in a way that's never before been possible.
What Exactly Is Blockchain, Anyway?
To understand the buzz around blockchain, it's necessary to grasp (at least loosely) how the technology works. Ordinary digital recordkeeping systems rely on a central administrator that acts as gatekeeper to a treasury of data; if you can sneak past the guard, you can often gain access to the entire hoard, and your intrusion may go undetected indefinitely. Blockchain, by contrast, employs a network of synchronized, replicated databases. Information is scattered among these nodes, rather than on a single server, and is exchanged through encrypted, peer-to-peer pathways. Each transaction is visible to every computer on the network, and must be approved by a majority in order to be successfully completed. Each batch of transactions, or "block," is date- and time-stamped, marked with the user's identity, and given a cryptographic code, which is posted to every node. These blocks form a "chain," preserved in an electronic ledger, that can be read by all users but can't be edited. Any unauthorized access, or attempt at tampering, can be quickly neutralized by these overlapping safeguards. Even if a hacker managed to break into the system, penetrating deeply would be extraordinarily difficult.
Because blockchain technology shares transaction records throughout a network, it could eliminate communication bottlenecks between different components of the healthcare system (primary care physicians, specialists, nurses, and so on). And because blockchain-based systems are designed to incorporate programs known as "smart contracts," which automate functions previously requiring human intervention, they could reduce dangerous slipups as well as tedious and costly paperwork. For example, when a patient gets a checkup, sees a specialist, and fills a prescription, all these actions could be automatically recorded on his or her electronic health record (EHR), checked for errors, submitted for billing, and entered on insurance claims—which could be adjudicated and reimbursed automatically as well. "Blockchain has the potential to remove a lot of intermediaries from existing workflows, whether digital or nondigital," says Kamaljit Behera, an industry analyst for the consulting firm Frost & Sullivan.
The possible upsides don't end there. By creating a detailed, comprehensive, and immutable timeline of medical transactions, blockchain-based recordkeeping could help providers gauge a patient's long-term health patterns in a way that's never before been possible. In addition to data entered by their caregivers, individuals could use app-based technologies or wearables to transmit other information to their records, such as diet, exercise, and sleep patterns, adding new depth to their medical portraits.
Many experts expect healthcare blockchain to take root more slowly in the U.S. than in nations with government-run national health services.
Smart contracts could also allow patients to specify who has access to their data. "If you get an MRI and want your orthopedist to see it, you can add him to your network instead of carrying a CD into his office," explains Andrew Lippman, associate director of the MIT Media Lab, who helped create a prototype healthcare blockchain system called MedRec that's currently being tested at Beth Israel Deaconess Hospital in Boston. "Or you might make a smart contract to allow your son or daughter to access your healthcare records if something happens to you." Another option: permitting researchers to analyze your data for scientific purposes, whether anonymously or with your name attached.
The Recent History, and Looking Ahead
Over the past two years, a crowd of startups has begun vying for a piece of the emerging healthcare blockchain market. Some, like PokitDok and Atlanta-based Patientory, plan to mint proprietary cryptocurrencies, which investors can buy in lieu of stock, medical providers may earn as a reward for achieving better outcomes, and patients might score for meeting wellness goals or participating in clinical trials. (Patientory's initial coin offering, or ICO, raised more than $7 million in three days.) Several fledgling healthcare-blockchain companies have found powerful corporate partners: Intel for Silicon Valley's PokitDok, Kaiser Permanente for Patientory, Philips for Los Angeles-based Gem Health. At least one established provider network, Change Healthcare, is developing blockchain-based systems of its own. Two months ago, Change launched what it calls the first "enterprise-scale" blockchain network in U.S. healthcare—a system to track insurance claim submissions and remittances.
No one, however, has set a roll-out date for a full-blown, blockchain-based EHR system in this country. "We have yet to see anything move from the pilot phase to some kind of production status," says Debbie Bucci, an IT architect in the federal government's Office of the National Coordinator for Health Information Technology. Indeed, many experts expect healthcare blockchain to take root more slowly here than in nations with government-run national health services. In America, a typical patient may have dealings with a family doctor who keeps everything on paper, an assortment of hospitals that use different EHR systems, and an insurer whose system for processing claims is separate from that of the healthcare providers. To help bridge these gaps, a consortium called the Hyperledger Healthcare Working Group (which includes many of the leading players in the field) is developing standard protocols for blockchain interoperability and other functions. Adding to the complexity is the federal Health Insurance and Portability Act (HIPAA), which governs who can access patient data and under what circumstances. "Healthcare blockchain is in a very nascent stage," says Behera. "Coming up with regulations and other guidelines, and achieving large-scale implementation, will take some time."
The ethical implications of buying and selling personal genomic data in an electronic marketplace are doubtless open to debate.
How long? Behera, like other analysts, estimates that relatively simple applications, such as revenue-cycle management systems, could become commonplace in the next five years. More ambitious efforts might reach fruition in a decade or so. But once the infrastructure for healthcare blockchain is fully established, its uses could go far beyond keeping better EHRs.
A handful of scientists and entrepreneurs are already working to develop one visionary application: managing genomic data. Last month, Harvard University geneticist George Church—one of the most influential figures in his discipline—launched a business called Nebula Genomics. It aims to set up an exchange in which individuals can use "Neptune tokens" to purchase DNA sequencing, which will be stored in the company's blockchain-based system; research groups will be able to pay clients for their data using the same cryptocurrency. Luna DNA, founded by a team of biotech veterans in San Diego, plans a similar service, as does a Moscow-based startup called the Zenome Project.
Hossein Rahnama, CEO of the mobile-tech company Flybits and director of research at the Ryerson Centre for Cloud and Context-Aware Computing in Toronto, envisions a more personalized way of sharing genomic data via blockchain. His firm is working with a U.S. insurance company to develop a service that would allow clients in their 20s and 30s to connect with people in their 70s or 80s with similar genomes. The young clients would learn how the elders' lifestyle choices had influenced their health, so that they could modify their own habits accordingly. "It's intergenerational wisdom-sharing," explains Rahnama, who is 38. "I would actually pay to be a part of that network."
The ethical implications of buying and selling personal genomic data in an electronic marketplace are doubtless open to debate. Such commerce could greatly expand the pool of subjects for research in many areas of medicine, enabling the kinds of breakthroughs that only Big Data can provide. Yet it could also lead millions to surrender the most private information of all—the secrets of their cells—to buyers with less benign intentions. The Dark Overlord, one might argue, could not hope for a more satisfying victory.
These scenarios, however, are pure conjecture. After the first web page was posted, in 1991, Lippman observes, "a whole universe developed that you couldn't have imagined on Day 1." The same, he adds, is likely true for healthcare blockchain. "Our vision is to make medical records useful for you and for society, and to give you more control over your own identity. Time will tell."
A movie still from the 1966 film "Fantastic Voyage"
In the 1966 movie "Fantastic Voyage," actress Raquel Welch and her submarine were shrunk to the size of a cell in order to eliminate a blood clot in a scientist's brain. Now, 55 years later, the scenario is becoming closer to reality.
California-based startup Bionaut Labs has developed a nanobot about the size of a grain of rice that's designed to transport medication to the exact location in the body where it's needed. If you think about it, the conventional way to deliver medicine makes little sense: A painkiller affects the entire body instead of just the arm that's hurting, and chemotherapy is flushed through all the veins instead of precisely targeting the tumor.
"Chemotherapy is delivered systemically," Bionaut-founder and CEO Michael Shpigelmacher says. "Often only a small percentage arrives at the location where it is actually needed."
But what if it was possible to send a tiny robot through the body to attack a tumor or deliver a drug at exactly the right location?
Several startups and academic institutes worldwide are working to develop such a solution but Bionaut Labs seems the furthest along in advancing its invention. "You can think of the Bionaut as a tiny screw that moves through the veins as if steered by an invisible screwdriver until it arrives at the tumor," Shpigelmacher explains. Via Zoom, he shares the screen of an X-ray machine in his Culver City lab to demonstrate how the half-transparent, yellowish device winds its way along the spine in the body. The nanobot contains a tiny but powerful magnet. The "invisible screwdriver" is an external magnetic field that rotates that magnet inside the device and gets it to move and change directions.
The current model has a diameter of less than a millimeter. Shpigelmacher's engineers could build the miniature vehicle even smaller but the current size has the advantage of being big enough to see with bare eyes. It can also deliver more medicine than a tinier version. In the Zoom demonstration, the micorobot is injected into the spine, not unlike an epidural, and pulled along the spine through an outside magnet until the Bionaut reaches the brainstem. Depending which organ it needs to reach, it could be inserted elsewhere, for instance through a catheter.
"The hope is that we can develop a vehicle to transport medication deep into the body," says Max Planck scientist Tian Qiu.
Imagine moving a screw through a steak with a magnet — that's essentially how the device works. But of course, the Bionaut is considerably different from an ordinary screw: "At the right location, we give a magnetic signal, and it unloads its medicine package," Shpigelmacher says.
To start, Bionaut Labs wants to use its device to treat Parkinson's disease and brain stem gliomas, a type of cancer that largely affects children and teenagers. About 300 to 400 young people a year are diagnosed with this type of tumor. Radiation and brain surgery risk damaging sensitive brain tissue, and chemotherapy often doesn't work. Most children with these tumors live less than 18 months. A nanobot delivering targeted chemotherapy could be a gamechanger. "These patients really don't have any other hope," Shpigelmacher says.
Of course, the main challenge of the developing such a device is guaranteeing that it's safe. Because tissue is so sensitive, any mistake could risk disastrous results. In recent years, Bionaut has tested its technology in dozens of healthy sheep and pigs with no major adverse effects. Sheep make a good stand-in for humans because their brains and spines are similar to ours.

The Bionaut device is about the size of a grain of rice.
Bionaut Labs
"As the Bionaut moves through brain tissue, it creates a transient track that heals within a few weeks," Shpigelmacher says. The company is hoping to be the first to test a nanobot in humans. In December 2022, it announced that a recent round of funding drew $43.2 million, for a total of 63.2 million, enabling more research and, if all goes smoothly, human clinical trials by early next year.
Once the technique has been perfected, further applications could include addressing other kinds of brain disorders that are considered incurable now, such as Alzheimer's or Huntington's disease. "Microrobots could serve as a bridgehead, opening the gateway to the brain and facilitating precise access of deep brain structure – either to deliver medication, take cell samples or stimulate specific brain regions," Shpigelmacher says.
Robot-assisted hybrid surgery with artificial intelligence is already used in state-of-the-art surgery centers, and many medical experts believe that nanorobotics will be the instrument of the future. In 2016, three scientists were awarded the Nobel Prize in Chemistry for their development of "the world's smallest machines," nano "elevators" and minuscule motors. Since then, the scientific experiments have progressed to the point where applicable devices are moving closer to actually being implemented.
Bionaut's technology was initially developed by a research team lead by Peer Fischer, head of the independent Micro Nano and Molecular Systems Lab at the Max Planck Institute for Intelligent Systems in Stuttgart, Germany. Fischer is considered a pioneer in the research of nano systems, which he began at Harvard University more than a decade ago. He and his team are advising Bionaut Labs and have licensed their technology to the company.
"The hope is that we can develop a vehicle to transport medication deep into the body," says Max Planck scientist Tian Qiu, who leads the cooperation with Bionaut Labs. He agrees with Shpigelmacher that the Bionaut's size is perfect for transporting medication loads and is researching potential applications for even smaller nanorobots, especially in the eye, where the tissue is extremely sensitive. "Nanorobots can sneak through very fine tissue without causing damage."
In "Fantastic Voyage," Raquel Welch's adventures inside the body of a dissident scientist let her swim through his veins into his brain, but her shrunken miniature submarine is attacked by antibodies; she has to flee through the nerves into the scientist's eye where she escapes into freedom on a tear drop. In reality, the exit in the lab is much more mundane. The Bionaut simply leaves the body through the same port where it entered. But apart from the dramatization, the "Fantastic Voyage" was almost prophetic, or, as Shpigelmacher says, "Science fiction becomes science reality."
This article was first published by Leaps.org on April 12, 2021.
How the Human Brain Project Built a Mind of its Own
In 2013, the Human Brain Project set out to build a realistic computer model of the brain over ten years. Now, experts are reflecting on HBP's achievements with an eye toward the future.
In 2009, neuroscientist Henry Markram gave an ambitious TED talk. “Our mission is to build a detailed, realistic computer model of the human brain,” he said, naming three reasons for this unmatched feat of engineering. One was because understanding the human brain was essential to get along in society. Another was because experimenting on animal brains could only get scientists so far in understanding the human ones. Third, medicines for mental disorders weren’t good enough. “There are two billion people on the planet that are affected by mental disorders, and the drugs that are used today are largely empirical,” Markram said. “I think that we can come up with very concrete solutions on how to treat disorders.”
Markram's arguments were very persuasive. In 2013, the European Commission launched the Human Brain Project, or HBP, as part of its Future and Emerging Technologies program. Viewed as Europe’s chance to try to win the “brain race” between the U.S., China, Japan, and other countries, the project received about a billion euros in funding with the goal to simulate the entire human brain on a supercomputer, or in silico, by 2023.
Now, after 10 years of dedicated neuroscience research, the HBP is coming to an end. As its many critics warned, it did not manage to build an entire human brain in silico. Instead, it achieved a multifaceted array of different goals, some of them unexpected.
Scholars have found that the project did help advance neuroscience more than some detractors initially expected, specifically in the area of brain simulations and virtual models. Using an interdisciplinary approach of combining technology, such as AI and digital simulations, with neuroscience, the HBP worked to gain a deeper understanding of the human brain’s complicated structure and functions, which in some cases led to novel treatments for brain disorders. Lastly, through online platforms, the HBP spearheaded a previously unmatched level of global neuroscience collaborations.
Simulating a human brain stirs up controversy
Right from the start, the project was plagued with controversy and condemnation. One of its prominent critics was Yves Fregnac, a professor in cognitive science at the Polytechnic Institute of Paris and research director at the French National Centre for Scientific Research. Fregnac argued in numerous articles that the HBP was overfunded based on proposals with unrealistic goals. “This new way of over-selling scientific targets, deeply aligned with what modern society expects from mega-sciences in the broad sense (big investment, big return), has been observed on several occasions in different scientific sub-fields,” he wrote in one of his articles, “before invading the field of brain sciences and neuromarketing.”
"A human brain model can simulate an experiment a million times for many different conditions, but the actual human experiment can be performed only once or a few times," said Viktor Jirsa, a professor at Aix-Marseille University.
Responding to such critiques, the HBP worked to restructure the effort in its early days with new leadership, organization, and goals that were more flexible and attainable. “The HBP got a more versatile, pluralistic approach,” said Viktor Jirsa, a professor at Aix-Marseille University and one of the HBP lead scientists. He believes that these changes fixed at least some of HBP’s issues. “The project has been on a very productive and scientifically fruitful course since then.”
After restructuring, the HBP became a European hub on brain research, with hundreds of scientists joining its growing network. The HBP created projects focused on various brain topics, from consciousness to neurodegenerative diseases. HBP scientists worked on complex subjects, such as mapping out the brain, combining neuroscience and robotics, and experimenting with neuromorphic computing, a computational technique inspired by the human brain structure and function—to name just a few.
Simulations advance knowledge and treatment options
In 2013, it seemed that bringing neuroscience into a digital age would be farfetched, but research within the HBP has made this achievable. The virtual maps and simulations various HBP teams create through brain imaging data make it easier for neuroscientists to understand brain developments and functions. The teams publish these models on the HBP’s EBRAINS online platform—one of the first to offer access to such data to neuroscientists worldwide via an open-source online site. “This digital infrastructure is backed by high-performance computers, with large datasets and various computational tools,” said Lucy Xiaolu Wang, an assistant professor in the Resource Economics Department at the University of Massachusetts Amherst, who studies the economics of the HBP. That means it can be used in place of many different types of human experimentation.
Jirsa’s team is one of many within the project that works on virtual brain models and brain simulations. Compiling patient data, Jirsa and his team can create digital simulations of different brain activities—and repeat these experiments many times, which isn’t often possible in surgeries on real brains. “A human brain model can simulate an experiment a million times for many different conditions,” Jirsa explained, “but the actual human experiment can be performed only once or a few times.” Using simulations also saves scientists and doctors time and money when looking at ways to diagnose and treat patients with brain disorders.

Compiling patient data, scientists can create digital simulations of different brain activities—and repeat these experiments many times.
The Human Brain Project
Simulations can help scientists get a full picture that otherwise is unattainable. “Another benefit is data completion,” added Jirsa, “in which incomplete data can be complemented by the model. In clinical settings, we can often measure only certain brain areas, but when linked to the brain model, we can enlarge the range of accessible brain regions and make better diagnostic predictions.”
With time, Jirsa’s team was able to move into patient-specific simulations. “We advanced from generic brain models to the ability to use a specific patient’s brain data, from measurements like MRI and others, to create individualized predictive models and simulations,” Jirsa explained. He and his team are working on this personalization technique to treat patients with epilepsy. According to the World Health Organization, about 50 million people worldwide suffer from epilepsy, a disorder that causes recurring seizures. While some epilepsy causes are known others remain an enigma, and many are hard to treat. For some patients whose epilepsy doesn’t respond to medications, removing part of the brain where seizures occur may be the only option. Understanding where in the patients’ brains seizures arise can give scientists a better idea of how to treat them and whether to use surgery versus medications.
“We apply such personalized models…to precisely identify where in a patient’s brain seizures emerge,” Jirsa explained. “This guides individual surgery decisions for patients for which surgery is the only treatment option.” He credits the HBP for the opportunity to develop this novel approach. “The personalization of our epilepsy models was only made possible by the Human Brain Project, in which all the necessary tools have been developed. Without the HBP, the technology would not be in clinical trials today.”
Personalized simulations can significantly advance treatments, predict the outcome of specific medical procedures and optimize them before actually treating patients. Jirsa is watching this happen firsthand in his ongoing research. “Our technology for creating personalized brain models is now used in a large clinical trial for epilepsy, funded by the French state, where we collaborate with clinicians in hospitals,” he explained. “We have also founded a spinoff company called VB Tech (Virtual Brain Technologies) to commercialize our personalized brain model technology and make it available to all patients.”
The Human Brain Project created a level of interconnectedness within the neuroscience research community that never existed before—a network not unlike the brain’s own.
Other experts believe it’s too soon to tell whether brain simulations could change epilepsy treatments. “The life cycle of developing treatments applicable to patients often runs over a decade,” Wang stated. “It is still too early to draw a clear link between HBP’s various project areas with patient care.” However, she admits that some studies built on the HBP-collected knowledge are already showing promise. “Researchers have used neuroscientific atlases and computational tools to develop activity-specific stimulation programs that enabled paraplegic patients to move again in a small-size clinical trial,” Wang said. Another intriguing study looked at simulations of Alzheimer’s in the brain to understand how it evolves over time.
Some challenges remain hard to overcome even with computer simulations. “The major challenge has always been the parameter explosion, which means that many different model parameters can lead to the same result,” Jirsa explained. An example of this parameter explosion could be two different types of neurodegenerative conditions, such as Parkinson’s and Huntington’s diseases. Both afflict the same area of the brain, the basal ganglia, which can affect movement, but are caused by two different underlying mechanisms. “We face the same situation in the living brain, in which a large range of diverse mechanisms can produce the same behavior,” Jirsa said. The simulations still have to overcome the same challenge.
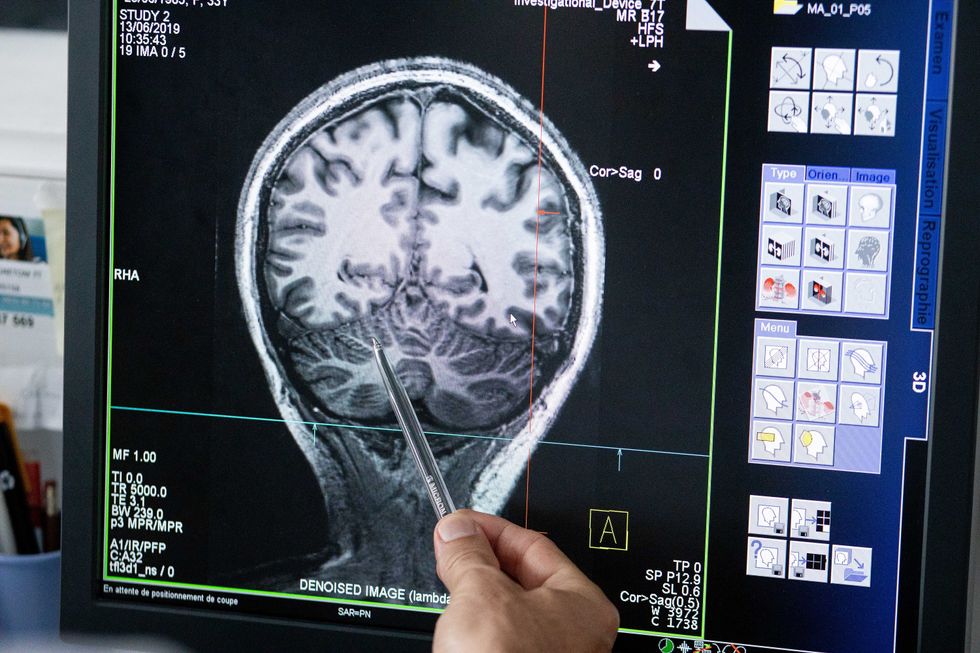
Understanding where in the patients’ brains seizures arise can give scientists a better idea of how to treat them and whether to use surgery versus medications.
The Human Brain Project
A network not unlike the brain’s own
Though the HBP will be closing this year, its legacy continues in various studies, spin-off companies, and its online platform, EBRAINS. “The HBP is one of the earliest brain initiatives in the world, and the 10-year long-term goal has united many researchers to collaborate on brain sciences with advanced computational tools,” Wang said. “Beyond the many research articles and projects collaborated on during the HBP, the online neuroscience research infrastructure EBRAINS will be left as a legacy even after the project ends.”
Those who worked within the HBP see the end of this project as the next step in neuroscience research. “Neuroscience has come closer to very meaningful applications through the systematic link with new digital technologies and collaborative work,” Jirsa stated. “In that way, the project really had a pioneering role.” It also created a level of interconnectedness within the neuroscience research community that never existed before—a network not unlike the brain’s own. “Interconnectedness is an important advance and prerequisite for progress,” Jirsa said. “The neuroscience community has in the past been rather fragmented and this has dramatically changed in recent years thanks to the Human Brain Project.”
According to its website, by 2023 HBP’s network counted over 500 scientists from over 123 institutions and 16 different countries, creating one of the largest multi-national research groups in the world. Even though the project hasn’t produced the in-silico brain as Markram envisioned it, the HBP created a communal mind with immense potential. “It has challenged us to think beyond the boundaries of our own laboratories,” Jirsa said, “and enabled us to go much further together than we could have ever conceived going by ourselves.”