Why Blindness Will Be the First Disorder Cured by Futuristic Treatments

A blind man with a cane goes for a walk at sunset. (© Prazis/Fotolia)
Stem cells and gene therapy were supposed to revolutionize biomedicine around the turn of the millennium and provide relief for desperate patients with incurable diseases. But for many, progress has been frustratingly slow. We still cannot, for example, regenerate damaged organs like a salamander regrows its tail, and genome engineering is more complicated than cutting and pasting letters in a word document.
"There are a number of things that make [the eye] ideal for new experimental therapies which are not true necessarily in other organs."
For blind people, however, the future of medicine is one step closer to reality. In December, the FDA approved the first gene therapy for an inherited disease—a mutation in the gene RPE65 that causes a rare form of blindness. Several clinical trials also show promise for treating various forms of retinal degeneration using stem cells.
"It's not surprising that the first gene therapy that was approved by the FDA was a therapy in the eye," says Bruce Conklin, a senior investigator at the San Francisco-based Gladstone Institutes, a nonprofit life science research organization, and a professor in the Medical Genetics and Molecular Pharmacology department at the University of California, San Francisco. "There are a number of things that make it ideal for new experimental therapies which are not true necessarily in other organs."
Physicians can easily see into the eye to check if a procedure worked or if it's causing problems. "The imaging technology within the eye is really unprecedented. You can't do this in someone's spinal cord or someone's brain cells or immune system," says Conklin, who is also deputy director of the Innovative Genomics Institute.
There's also a built-in control: researchers can test an intervention on one eye first. What's more, if something goes wrong, the risk of mortality is low, especially when compared to experimenting on the heart or brain. Most types of blindness are currently incurable, so the risk-to-reward ratio for patients is high. If a problem arises with the treatment their eyesight could get worse, but if they do nothing their vision will likely decline anyway. And if the treatment works, they may be able to see for the first time in years.
Gene Therapy
An additional appeal for testing gene therapy in the eye is the low risk for off-target effects, in which genome edits could result in unintended changes to other genes or in other cell types. There are a number of genes that are solely expressed in the eye and not in any other part of the body. Manipulating those genes will only affect cells in the eye, so concerns about the impact on other organs are minimal.
Ninety-three percent of patients who received the injection had improved vision just one month after treatment.
RPE65 is one such gene. It creates an enzyme that helps the eye convert light into an electrical signal that travels back to the brain. Patients with the mutation don't produce the enzyme, so visual signals are not processed. However, the retinal cells in the eye remain healthy for years; if you can restore the missing enzyme you can restore vision.
The newly approved therapy, developed by Spark Therapeutics, uses a modified virus to deliver RPE65 into the eye. A retinal surgeon injects the virus, which has been specially engineered to remove its disease-causing genes and instead carry the correct RPE65 gene, into the retina. There, it is sucked up by retinal pigment epithelial (RPE) cells. The RPE cells are a particularly good target for injection because their job is to eat up and recycle rogue particles. Once inside the cell, the virus slips into the nucleus and releases the DNA. The RPE65 gene then goes to work, using the cell's normal machinery to produce the needed enzyme.
In the most recent clinical trial, 93 percent of patients who received the injection—who range in age from 4 to 44—had improved vision just one month after treatment. So far, the benefits have lasted at least two years.
"It's an exciting time for this class of diseases, where these people have really not had treatments," says Spark president and co-founder, Katherine High. "[Gene therapy] affords the possibility of treatment for diseases that heretofore other classes of therapeutics really have not been able to help."
Stem Cells
Another benefit of the eye is its immune privilege. In order to let light in, the eye must remain transparent. As a result, its immune system is dampened so that it won't become inflamed if outside particles get in. This means the eye is much less likely to reject cell transplants, so patients do not need to take immunosuppressant drugs.
One study generating buzz is a clinical trial in Japan that is the first and, so far, only test of induced pluripotent stem cells in the eye.
Henry Klassen, an assistant professor at UC Irvine, is taking advantage of the eye's immune privilege to transplant retinal progenitor cells into the eye to treat retinitis pigmentosa, an inherited disease affecting about 1 in 4000 people that eventually causes the retina to degenerate. The disease can stem from dozens of different genetic mutations, but the result is the same: RPE cells die off over the course of a few decades, leaving the patient blind by middle age. It is currently incurable.
Retinal progenitor cells are baby retinal cells that develop naturally from stem cells and will turn into one of several types of adult retinal cells. When transplanted into a patient's eye, the progenitor cells don't replace the lost retinal cells, but they do secrete proteins and enzymes essential for eye health.
"At the stage we get the retinal tissue it's immature," says Klassen. "They still have some flexibility in terms of which mature cells they can turn into. It's that inherent flexibility that gives them a lot of power when they're put in the context of a diseased retina."
Klassen's spin-off company, jCyte, sponsored the clinical trial with support from the California Institute for Regenerative Medicine. The results from the initial study haven't been published yet, but Klassen says he considers it a success. JCyte is now embarking on a phase two trial to assess improvements in vision after the treatment, which will wrap up in 2021.
Another study generating buzz is a clinical trial in Japan that is the first and, so far, only test of induced pluripotent stem cells (iPSC) in the eye. iPSC are created by reprogramming a patient's own skin cells into stem cells, circumventing any controversy around embryonic stem cell sources. In the trial, led by Masayo Takahashi at RIKEN, the scientists transplant retinal pigment epithelial cells created from iPSC into the retinas of patients with age-related macular degeneration. The first woman to receive the treatment is doing well, and her vision is stable. However, the second patient suffered a swollen retina as a result of the surgery. Despite this recent setback, Takahashi said last week that the trial would continue.
Botched Jobs
Although recent studies have provided patients with renewed hope, the field has not been without mishap. Most notably, an article in the New England Journal of Medicine last March described three patients who experienced severe side effects after receiving stem cell injections from a Florida clinic to treat age-related macular degeneration. Following the initial article, other reports came out about similar botched treatments. Lawsuits have been filed against US Stem Cell, the clinic that conducted the procedure, and the FDA sent them a warning letter with a long list of infractions.
"One red flag is that the clinics charge patients to take part in the treatment—something extremely unusual for legitimate clinical trials."
Ajay Kuriyan, an ophthalmologist and retinal specialist at the University of Rochester who wrote the paper, says that because details about the Florida trial are scarce, it's hard to say why the treatment caused the adverse reaction. His guess is that the stem cells were poorly prepared and not up to clinical standards.
Klassen agrees that small clinics like US Stem Cell do not offer the same caliber of therapy as larger clinical trials. "It's not the same cells and it's not the same technique and it's not the same supervision and it's not under FDA auspices. It's just not the same thing," he says. "Unfortunately, to the patient it might sound the same, and that's the tragedy for me."
For patients who are interested in joining a trial, Kuriyan listed a few things to watch out for. "One red flag is that the clinics charge patients to take part in the treatment—something extremely unusual for legitimate clinical trials," he says. "Another big red flag is doing the procedure in both eyes" at the same time. Third, if the only treatment offered is cell therapy. "These clinics tend to be sort of stand-alone clinics, and that's not very common for an actual big research study of this scale."
Despite the recent scandal, Klassen hopes that the success of his trial and others will continue to push the field forward. "It just takes so many decades to move this stuff along, even when you're trying to simplify it as much as possible," he says. "With all the heavy lifting that's been done, I hope the world's got the patience to get this through."
A movie still from the 1966 film "Fantastic Voyage"
In the 1966 movie "Fantastic Voyage," actress Raquel Welch and her submarine were shrunk to the size of a cell in order to eliminate a blood clot in a scientist's brain. Now, 55 years later, the scenario is becoming closer to reality.
California-based startup Bionaut Labs has developed a nanobot about the size of a grain of rice that's designed to transport medication to the exact location in the body where it's needed. If you think about it, the conventional way to deliver medicine makes little sense: A painkiller affects the entire body instead of just the arm that's hurting, and chemotherapy is flushed through all the veins instead of precisely targeting the tumor.
"Chemotherapy is delivered systemically," Bionaut-founder and CEO Michael Shpigelmacher says. "Often only a small percentage arrives at the location where it is actually needed."
But what if it was possible to send a tiny robot through the body to attack a tumor or deliver a drug at exactly the right location?
Several startups and academic institutes worldwide are working to develop such a solution but Bionaut Labs seems the furthest along in advancing its invention. "You can think of the Bionaut as a tiny screw that moves through the veins as if steered by an invisible screwdriver until it arrives at the tumor," Shpigelmacher explains. Via Zoom, he shares the screen of an X-ray machine in his Culver City lab to demonstrate how the half-transparent, yellowish device winds its way along the spine in the body. The nanobot contains a tiny but powerful magnet. The "invisible screwdriver" is an external magnetic field that rotates that magnet inside the device and gets it to move and change directions.
The current model has a diameter of less than a millimeter. Shpigelmacher's engineers could build the miniature vehicle even smaller but the current size has the advantage of being big enough to see with bare eyes. It can also deliver more medicine than a tinier version. In the Zoom demonstration, the micorobot is injected into the spine, not unlike an epidural, and pulled along the spine through an outside magnet until the Bionaut reaches the brainstem. Depending which organ it needs to reach, it could be inserted elsewhere, for instance through a catheter.
"The hope is that we can develop a vehicle to transport medication deep into the body," says Max Planck scientist Tian Qiu.
Imagine moving a screw through a steak with a magnet — that's essentially how the device works. But of course, the Bionaut is considerably different from an ordinary screw: "At the right location, we give a magnetic signal, and it unloads its medicine package," Shpigelmacher says.
To start, Bionaut Labs wants to use its device to treat Parkinson's disease and brain stem gliomas, a type of cancer that largely affects children and teenagers. About 300 to 400 young people a year are diagnosed with this type of tumor. Radiation and brain surgery risk damaging sensitive brain tissue, and chemotherapy often doesn't work. Most children with these tumors live less than 18 months. A nanobot delivering targeted chemotherapy could be a gamechanger. "These patients really don't have any other hope," Shpigelmacher says.
Of course, the main challenge of the developing such a device is guaranteeing that it's safe. Because tissue is so sensitive, any mistake could risk disastrous results. In recent years, Bionaut has tested its technology in dozens of healthy sheep and pigs with no major adverse effects. Sheep make a good stand-in for humans because their brains and spines are similar to ours.

The Bionaut device is about the size of a grain of rice.
Bionaut Labs
"As the Bionaut moves through brain tissue, it creates a transient track that heals within a few weeks," Shpigelmacher says. The company is hoping to be the first to test a nanobot in humans. In December 2022, it announced that a recent round of funding drew $43.2 million, for a total of 63.2 million, enabling more research and, if all goes smoothly, human clinical trials by early next year.
Once the technique has been perfected, further applications could include addressing other kinds of brain disorders that are considered incurable now, such as Alzheimer's or Huntington's disease. "Microrobots could serve as a bridgehead, opening the gateway to the brain and facilitating precise access of deep brain structure – either to deliver medication, take cell samples or stimulate specific brain regions," Shpigelmacher says.
Robot-assisted hybrid surgery with artificial intelligence is already used in state-of-the-art surgery centers, and many medical experts believe that nanorobotics will be the instrument of the future. In 2016, three scientists were awarded the Nobel Prize in Chemistry for their development of "the world's smallest machines," nano "elevators" and minuscule motors. Since then, the scientific experiments have progressed to the point where applicable devices are moving closer to actually being implemented.
Bionaut's technology was initially developed by a research team lead by Peer Fischer, head of the independent Micro Nano and Molecular Systems Lab at the Max Planck Institute for Intelligent Systems in Stuttgart, Germany. Fischer is considered a pioneer in the research of nano systems, which he began at Harvard University more than a decade ago. He and his team are advising Bionaut Labs and have licensed their technology to the company.
"The hope is that we can develop a vehicle to transport medication deep into the body," says Max Planck scientist Tian Qiu, who leads the cooperation with Bionaut Labs. He agrees with Shpigelmacher that the Bionaut's size is perfect for transporting medication loads and is researching potential applications for even smaller nanorobots, especially in the eye, where the tissue is extremely sensitive. "Nanorobots can sneak through very fine tissue without causing damage."
In "Fantastic Voyage," Raquel Welch's adventures inside the body of a dissident scientist let her swim through his veins into his brain, but her shrunken miniature submarine is attacked by antibodies; she has to flee through the nerves into the scientist's eye where she escapes into freedom on a tear drop. In reality, the exit in the lab is much more mundane. The Bionaut simply leaves the body through the same port where it entered. But apart from the dramatization, the "Fantastic Voyage" was almost prophetic, or, as Shpigelmacher says, "Science fiction becomes science reality."
This article was first published by Leaps.org on April 12, 2021.
How the Human Brain Project Built a Mind of its Own
In 2013, the Human Brain Project set out to build a realistic computer model of the brain over ten years. Now, experts are reflecting on HBP's achievements with an eye toward the future.
In 2009, neuroscientist Henry Markram gave an ambitious TED talk. “Our mission is to build a detailed, realistic computer model of the human brain,” he said, naming three reasons for this unmatched feat of engineering. One was because understanding the human brain was essential to get along in society. Another was because experimenting on animal brains could only get scientists so far in understanding the human ones. Third, medicines for mental disorders weren’t good enough. “There are two billion people on the planet that are affected by mental disorders, and the drugs that are used today are largely empirical,” Markram said. “I think that we can come up with very concrete solutions on how to treat disorders.”
Markram's arguments were very persuasive. In 2013, the European Commission launched the Human Brain Project, or HBP, as part of its Future and Emerging Technologies program. Viewed as Europe’s chance to try to win the “brain race” between the U.S., China, Japan, and other countries, the project received about a billion euros in funding with the goal to simulate the entire human brain on a supercomputer, or in silico, by 2023.
Now, after 10 years of dedicated neuroscience research, the HBP is coming to an end. As its many critics warned, it did not manage to build an entire human brain in silico. Instead, it achieved a multifaceted array of different goals, some of them unexpected.
Scholars have found that the project did help advance neuroscience more than some detractors initially expected, specifically in the area of brain simulations and virtual models. Using an interdisciplinary approach of combining technology, such as AI and digital simulations, with neuroscience, the HBP worked to gain a deeper understanding of the human brain’s complicated structure and functions, which in some cases led to novel treatments for brain disorders. Lastly, through online platforms, the HBP spearheaded a previously unmatched level of global neuroscience collaborations.
Simulating a human brain stirs up controversy
Right from the start, the project was plagued with controversy and condemnation. One of its prominent critics was Yves Fregnac, a professor in cognitive science at the Polytechnic Institute of Paris and research director at the French National Centre for Scientific Research. Fregnac argued in numerous articles that the HBP was overfunded based on proposals with unrealistic goals. “This new way of over-selling scientific targets, deeply aligned with what modern society expects from mega-sciences in the broad sense (big investment, big return), has been observed on several occasions in different scientific sub-fields,” he wrote in one of his articles, “before invading the field of brain sciences and neuromarketing.”
"A human brain model can simulate an experiment a million times for many different conditions, but the actual human experiment can be performed only once or a few times," said Viktor Jirsa, a professor at Aix-Marseille University.
Responding to such critiques, the HBP worked to restructure the effort in its early days with new leadership, organization, and goals that were more flexible and attainable. “The HBP got a more versatile, pluralistic approach,” said Viktor Jirsa, a professor at Aix-Marseille University and one of the HBP lead scientists. He believes that these changes fixed at least some of HBP’s issues. “The project has been on a very productive and scientifically fruitful course since then.”
After restructuring, the HBP became a European hub on brain research, with hundreds of scientists joining its growing network. The HBP created projects focused on various brain topics, from consciousness to neurodegenerative diseases. HBP scientists worked on complex subjects, such as mapping out the brain, combining neuroscience and robotics, and experimenting with neuromorphic computing, a computational technique inspired by the human brain structure and function—to name just a few.
Simulations advance knowledge and treatment options
In 2013, it seemed that bringing neuroscience into a digital age would be farfetched, but research within the HBP has made this achievable. The virtual maps and simulations various HBP teams create through brain imaging data make it easier for neuroscientists to understand brain developments and functions. The teams publish these models on the HBP’s EBRAINS online platform—one of the first to offer access to such data to neuroscientists worldwide via an open-source online site. “This digital infrastructure is backed by high-performance computers, with large datasets and various computational tools,” said Lucy Xiaolu Wang, an assistant professor in the Resource Economics Department at the University of Massachusetts Amherst, who studies the economics of the HBP. That means it can be used in place of many different types of human experimentation.
Jirsa’s team is one of many within the project that works on virtual brain models and brain simulations. Compiling patient data, Jirsa and his team can create digital simulations of different brain activities—and repeat these experiments many times, which isn’t often possible in surgeries on real brains. “A human brain model can simulate an experiment a million times for many different conditions,” Jirsa explained, “but the actual human experiment can be performed only once or a few times.” Using simulations also saves scientists and doctors time and money when looking at ways to diagnose and treat patients with brain disorders.

Compiling patient data, scientists can create digital simulations of different brain activities—and repeat these experiments many times.
The Human Brain Project
Simulations can help scientists get a full picture that otherwise is unattainable. “Another benefit is data completion,” added Jirsa, “in which incomplete data can be complemented by the model. In clinical settings, we can often measure only certain brain areas, but when linked to the brain model, we can enlarge the range of accessible brain regions and make better diagnostic predictions.”
With time, Jirsa’s team was able to move into patient-specific simulations. “We advanced from generic brain models to the ability to use a specific patient’s brain data, from measurements like MRI and others, to create individualized predictive models and simulations,” Jirsa explained. He and his team are working on this personalization technique to treat patients with epilepsy. According to the World Health Organization, about 50 million people worldwide suffer from epilepsy, a disorder that causes recurring seizures. While some epilepsy causes are known others remain an enigma, and many are hard to treat. For some patients whose epilepsy doesn’t respond to medications, removing part of the brain where seizures occur may be the only option. Understanding where in the patients’ brains seizures arise can give scientists a better idea of how to treat them and whether to use surgery versus medications.
“We apply such personalized models…to precisely identify where in a patient’s brain seizures emerge,” Jirsa explained. “This guides individual surgery decisions for patients for which surgery is the only treatment option.” He credits the HBP for the opportunity to develop this novel approach. “The personalization of our epilepsy models was only made possible by the Human Brain Project, in which all the necessary tools have been developed. Without the HBP, the technology would not be in clinical trials today.”
Personalized simulations can significantly advance treatments, predict the outcome of specific medical procedures and optimize them before actually treating patients. Jirsa is watching this happen firsthand in his ongoing research. “Our technology for creating personalized brain models is now used in a large clinical trial for epilepsy, funded by the French state, where we collaborate with clinicians in hospitals,” he explained. “We have also founded a spinoff company called VB Tech (Virtual Brain Technologies) to commercialize our personalized brain model technology and make it available to all patients.”
The Human Brain Project created a level of interconnectedness within the neuroscience research community that never existed before—a network not unlike the brain’s own.
Other experts believe it’s too soon to tell whether brain simulations could change epilepsy treatments. “The life cycle of developing treatments applicable to patients often runs over a decade,” Wang stated. “It is still too early to draw a clear link between HBP’s various project areas with patient care.” However, she admits that some studies built on the HBP-collected knowledge are already showing promise. “Researchers have used neuroscientific atlases and computational tools to develop activity-specific stimulation programs that enabled paraplegic patients to move again in a small-size clinical trial,” Wang said. Another intriguing study looked at simulations of Alzheimer’s in the brain to understand how it evolves over time.
Some challenges remain hard to overcome even with computer simulations. “The major challenge has always been the parameter explosion, which means that many different model parameters can lead to the same result,” Jirsa explained. An example of this parameter explosion could be two different types of neurodegenerative conditions, such as Parkinson’s and Huntington’s diseases. Both afflict the same area of the brain, the basal ganglia, which can affect movement, but are caused by two different underlying mechanisms. “We face the same situation in the living brain, in which a large range of diverse mechanisms can produce the same behavior,” Jirsa said. The simulations still have to overcome the same challenge.
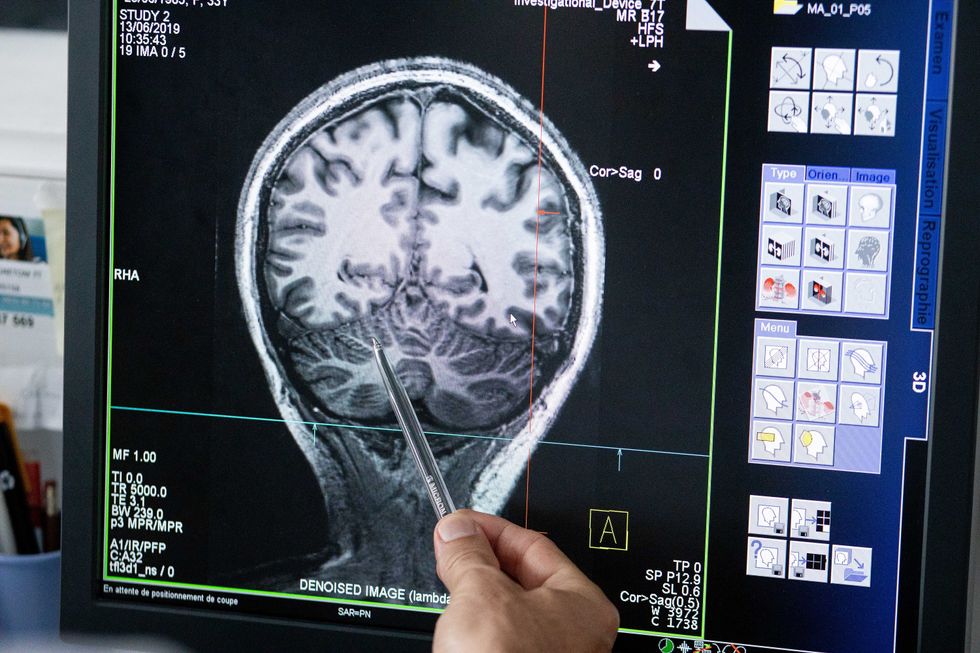
Understanding where in the patients’ brains seizures arise can give scientists a better idea of how to treat them and whether to use surgery versus medications.
The Human Brain Project
A network not unlike the brain’s own
Though the HBP will be closing this year, its legacy continues in various studies, spin-off companies, and its online platform, EBRAINS. “The HBP is one of the earliest brain initiatives in the world, and the 10-year long-term goal has united many researchers to collaborate on brain sciences with advanced computational tools,” Wang said. “Beyond the many research articles and projects collaborated on during the HBP, the online neuroscience research infrastructure EBRAINS will be left as a legacy even after the project ends.”
Those who worked within the HBP see the end of this project as the next step in neuroscience research. “Neuroscience has come closer to very meaningful applications through the systematic link with new digital technologies and collaborative work,” Jirsa stated. “In that way, the project really had a pioneering role.” It also created a level of interconnectedness within the neuroscience research community that never existed before—a network not unlike the brain’s own. “Interconnectedness is an important advance and prerequisite for progress,” Jirsa said. “The neuroscience community has in the past been rather fragmented and this has dramatically changed in recent years thanks to the Human Brain Project.”
According to its website, by 2023 HBP’s network counted over 500 scientists from over 123 institutions and 16 different countries, creating one of the largest multi-national research groups in the world. Even though the project hasn’t produced the in-silico brain as Markram envisioned it, the HBP created a communal mind with immense potential. “It has challenged us to think beyond the boundaries of our own laboratories,” Jirsa said, “and enabled us to go much further together than we could have ever conceived going by ourselves.”